Dentine Hypersensitivity
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Glossary for Narrative Writing
Periodontal Assessment and Treatment Planning Gingival description Color: o pink o erythematous o cyanotic o racial pigmentation o metallic pigmentation o uniformity Contour: o recession o clefts o enlarged papillae o cratered papillae o blunted papillae o highly rolled o bulbous o knife-edged o scalloped o stippled Consistency: o firm o edematous o hyperplastic o fibrotic Band of gingiva: o amount o quality o location o treatability Bleeding tendency: o sulcus base, lining o gingival margins Suppuration Sinus tract formation Pocket depths Pseudopockets Frena Pain Other pathology Dental Description Defective restorations: o overhangs o open contacts o poor contours Fractured cusps 1 ww.links2success.biz [email protected] 914-303-6464 Caries Deposits: o Type . plaque . calculus . stain . matera alba o Location . supragingival . subgingival o Severity . mild . moderate . severe Wear facets Percussion sensitivity Tooth vitality Attrition, erosion, abrasion Occlusal plane level Occlusion findings Furcations Mobility Fremitus Radiographic findings Film dates Crown:root ratio Amount of bone loss o horizontal; vertical o localized; generalized Root length and shape Overhangs Bulbous crowns Fenestrations Dehiscences Tooth resorption Retained root tips Impacted teeth Root proximities Tilted teeth Radiolucencies/opacities Etiologic factors Local: o plaque o calculus o overhangs 2 ww.links2success.biz [email protected] 914-303-6464 o orthodontic apparatus o open margins o open contacts o improper -

Importance of Chlorhexidine in Maintaining Periodontal Health
International Journal of Dentistry Research 2016; 1(1): 31-33 Review Article Importance of Chlorhexidine in Maintaining Periodontal IJDR 2016; 1(1): 31-33 December Health © 2016, All rights reserved www.dentistryscience.com Dr. Manpreet Kaur*1, Dr. Krishan Kumar1 1 Department of Periodontics, Post Graduate Institute of Dental Sciences, Rohtak-124001, Haryana, India Abstract Plaque is responsible for periodontal diseases. In order to prevent occurrence and progression of periodontal disease, removal of plaque becomes important. Mechanical tooth cleaning aids such as toothbrushes, dental floss, interdental brushes are used for removal of plaque. However, in some cases, chemical agents are used as an adjunct to mechanical methods to facilitate plaque control and prevent gingivitis. Chlorhexidine (CHX) mouthwash is the most commonly used and is considered as gold standard chemical agent. In this review, mechanism of action and other properties of CHX are discussed. Keywords: Plaque, Chemical agents, Chlorhexidine (CHX). INTRODUCTION Dental plaque is primary etiologic factor responsible for gingivitis and periodontitis [1]. Mechanical plaque control using toothbrushes, interdental brushes, dental floss prevent occurrence of gingivitis. However, in majority of population, mechanical methods of plaque control are ineffective due to less time spent[2] for plaque removal and lack of consistency. These limitations necessitate use of chemical plaque control agents as an adjunct to mechanical plaque control. Among various chemical agents, chlorhexidine (CHX) is considered to be a gold standard chemical agent for plaque control. Its structural formula consists of two symmetric 4-chlorophenyl rings and two biguanide groups connected by a central hexamethylene chain. Mechanism of action for CHX CHX is bactericidal and is effective against gram-positive bacteria, gram-negative bacteria and yeast organisms. -

Probiotic Alternative to Chlorhexidine in Periodontal Therapy: Evaluation of Clinical and Microbiological Parameters
microorganisms Article Probiotic Alternative to Chlorhexidine in Periodontal Therapy: Evaluation of Clinical and Microbiological Parameters Andrea Butera , Simone Gallo * , Carolina Maiorani, Domenico Molino, Alessandro Chiesa, Camilla Preda, Francesca Esposito and Andrea Scribante * Section of Dentistry–Department of Clinical, Surgical, Diagnostic and Paediatric Sciences, University of Pavia, 27100 Pavia, Italy; [email protected] (A.B.); [email protected] (C.M.); [email protected] (D.M.); [email protected] (A.C.); [email protected] (C.P.); [email protected] (F.E.) * Correspondence: [email protected] (S.G.); [email protected] (A.S.) Abstract: Periodontitis consists of a progressive destruction of tooth-supporting tissues. Considering that probiotics are being proposed as a support to the gold standard treatment Scaling-and-Root- Planing (SRP), this study aims to assess two new formulations (toothpaste and chewing-gum). 60 patients were randomly assigned to three domiciliary hygiene treatments: Group 1 (SRP + chlorhexidine-based toothpaste) (control), Group 2 (SRP + probiotics-based toothpaste) and Group 3 (SRP + probiotics-based toothpaste + probiotics-based chewing-gum). At baseline (T0) and after 3 and 6 months (T1–T2), periodontal clinical parameters were recorded, along with microbiological ones by means of a commercial kit. As to the former, no significant differences were shown at T1 or T2, neither in controls for any index, nor in the experimental -

June 18, 2013 8:30 Am – 11:30 Am
Tuesday – June 18, 2013 8:30 am – 11:30 am Poster Abstracts – Tuesday, June 18, 2013 #1 ORAL LESIONS AS THE PRESENTING MANIFESTATION OF CROHN'S DISEASE V Woo, E Herschaft, J Wang U of Nevada, Las Vegas Crohn’s disease (CD) is an immune-mediated disorder of the gastrointestinal tract which together with ulcerative colitis, comprise the two major types of inflammatory bowel disease (IBD). The underlying etiology has been attributed to defects in mucosal immunity and the intestinal epithelial barrier in a genetically susceptible host, resulting in an inappropriate inflammatory response to intestinal microbes. The lesions of CD can affect any region of the alimentary tract as well as extraintestinal sites such as the skin, joints and eyes. The most common presenting symptoms are periumbilical pain and diarrhea associated with fevers, malaise and anemia. Oral involvement has been termed oral CD and may manifest as lip swelling, cobblestoned mucosa, mucogingivitis and linear ulcerations and fissures. Oral lesions may precede gastrointestinal involvement and can serve as early markers of CD. We describe a 6-year-old male who presented for evaluation of multifocal gingival erythema and swellings. His medical history was unremarkable for gastrointestinal disorders or distress. Histopathologic examination showed multiple well-formed granulomas that were negative for special stains and foreign body material. A diagnosis of granulomatous gingivitis was rendered. The patient was advised to seek consultation with a pediatric gastroenterologist and following colonoscopy, was diagnosed with early stage CD. Timely recognition of the oral manifestations of CD is critical because only a minority of patients will continue to exhibit CD-specific oral lesions at follow-up. -
Sensitive Teeth.Qxp
Sensitive teeth may be a warning of more serious problems Do You Have Sensitive Teeth? If you have a common problem called “sensitive teeth,” a sip of iced tea or a cup of hot cocoa, the sudden intake of cold air or pressure from your toothbrush may be painful. Sensitive teeth can be experienced at any age as a momentary slight twinge to long-term severe discomfort. It is important to consult your dentist because sensitive teeth may be an early warning sign of more serious dental problems. Understanding Tooth Structure. What Causes Sensitive Teeth? To better understand how sensitivity There can be many causes for sensitive develops, we need to consider the teeth. Cavities, fractured teeth, worn tooth composition of tooth structure. The crown- enamel, cracked teeth, exposed tooth root, the part of the tooth that is most visible- gum recession or periodontal disease may has a tough, protective jacket of enamel, be causing the problem. which is an extremely strong substance. Below the gum line, a layer of cementum Periodontal disease is an infection of the protects the tooth root. Underneath the gums and bone that support the teeth. If left enamel and cementum is dentin. untreated, it can progress until bone and other supporting tissues are destroyed. This Dentin is a part of the tooth that contains can leave the root surfaces of teeth exposed tiny tubes. When dentin loses its and may lead to tooth sensitivity. protective covering and is exposed, these small tubes permit heat, cold, Brushing incorrectly or too aggressively may certain types of foods or pressure to injure your gums and can also cause tooth stimulate nerves and cells inside of roots to be exposed. -

Dental Cementum Reviewed: Development, Structure, Composition, Regeneration and Potential Functions
Braz J Oral Sci. January/March 2005 - Vol.4 - Number 12 Dental cementum reviewed: development, structure, composition, regeneration and potential functions Patricia Furtado Gonçalves 1 Enilson Antonio Sallum 1 Abstract Antonio Wilson Sallum 1 This article reviews developmental and structural characteristics of Márcio Zaffalon Casati 1 cementum, a unique avascular mineralized tissue covering the root Sérgio de Toledo 1 surface that forms the interface between root dentin and periodontal Francisco Humberto Nociti Junior 1 ligament. Besides describing the types of cementum and 1 Dept. of Prosthodontics and Periodontics, cementogenesis, attention is given to recent advances in scientific Division of Periodontics, School of Dentistry understanding of the molecular and cellular aspects of the formation at Piracicaba - UNICAMP, Piracicaba, São and regeneration of cementum. The understanding of the mechanisms Paulo, Brazil. involved in the dynamic of this tissue should allow for the development of new treatment strategies concerning the approach of the root surface affected by periodontal disease and periodontal regeneration techniques. Received for publication: October 01, 2004 Key Words: Accepted: December 17, 2004 dental cementum, review Correspondence to: Francisco H. Nociti Jr. Av. Limeira 901 - Caixa Postal: 052 - CEP: 13414-903 - Piracicaba - S.P. - Brazil Tel: ++ 55 19 34125298 Fax: ++ 55 19 3412 5218 E-mail: [email protected] 651 Braz J Oral Sci. 4(12): 651-658 Dental cementum reviewed: development, structure, composition, regeneration and potential functions Introduction junction (Figure 1). The areas and location of acellular Cementum is an avascular mineralized tissue covering the afibrillar cementum vary from tooth to tooth and along the entire root surface. Due to its intermediary position, forming cementoenamel junction of the same tooth6-9. -

Periodontal Practice Patterns
PERIODONTAL PRACTICE PATTERNS A Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of the Ohio State University By Janel Kimberlay Yu, D.D.S. Graduate Program in Dentistry The Ohio State University 2010 Dissertation Committee: Dr. Angelo Mariotti, Advisor Dr. Jed Jacobson Dr. Eric Seiber Copyright by Janel Kimberlay Yu 2010 Abstract Background: Differences in the rates of dental services between geographic regions are important since major discrepancies in practice patterns may suggest an absence of evidence-based clinical information leading to numerous treatment plans for similar dental problems and the misallocation of limited resources. Variations in dental care to patients may result from characteristics of the periodontist. Insurance claims data in this study were compared to the characteristics of periodontal providers to determine if variations in practice patterns exist. Methods: Claims data, between 2000-2009 from Delta Dental of Ohio, Michigan, Indiana, New Mexico, and Tennessee, were examined to analyze the practice patterns of 351 periodontists. For each provider, the average number of select CDT periodontal codes (4000-4999), implants (6010), and extractions (7140) were calculated over two time periods in relation to provider variable, including state, urban versus rural area, gender, experience, location of training, and membership in organized dentistry. Descriptive statistics were performed to depict the data using measures of central tendency and measures of dispersion. ii Results: Differences in periodontal procedures were present across states. Although the most common surgical procedure in the study period was osseous surgery, greater increases over time were observed in regenerative procedures (bone grafts, biologics, GTR) when compared to osseous surgery. -

The Cementum: Its Role in Periodontal Health and Disease*
THE JOURNAL OF PERIODONTOLOGY JULY, NINETEEN HUNDRED SIXTY ONE The Cementum: Its Role In Periodontal Health and Disease* by DONALD A. KERR, D.D.S., M.S.,** Ann Arbor, Michigan HE cementum is a specialized calcified tissue of mesenchymal origin which provides for the attachment of the periodontal fibers to the surface of the Troot. It consists of 45 to 50 per cent inorganic material and 50 to 55 per cent organic material with the inorganic material in a hydroxyl apatite structure. The primary cementum is formed initially by appositional growth from the dental sac and later from the periodontal membrane under the influence of cementoblasts. It is formed in laminated layers with the incorporation of Sharpey's fibers into a fibrillar matrix which undergoes calcification. Cementum deposition is a Continuous process throughout life with new cementum being deposited over the old cemental surface. Cementum is formed by the organiza• tion of collagen fibrils which are cemented together by a matrix produced by the polymerization of mucopolysaccharides. This material is designated as cementoid and becomes mature cementum upon calcification. The significance of the continuous deposition of cementum has received various interpretations. 1. Continuous deposition of cementum is necessary for the reattachment of periodontal fibers which have been destroyed or which require reorientation due to change in position of teeth. It is logical that there should be a continuous deposition of cementum because it is doubtful that the initial fibers are retained throughout the life of the tooth, and therefore new fibers must be continually formed and attached by new cementum. -

Abfraction Myth Or Reality
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861. Volume 13, Issue 2 Ver. III. (Feb. 2014), PP 70-73 www.iosrjournals.org Abfraction Myth or Reality Dr Pragya Tripathi, Dr Deepak Chopra, Dr Sukhchain Bagga Sr Lecturer Dept of Periodontics, Inderprastha Dental College, Sahibabad, Ghaziabad (UP) India. Reader Dept of Periodontics, Inderprastha Dental College, Sahibabad, Ghaziabad (UP) India. Reader Dept of Periodontics, Inderprastha Dental College, Sahibabad, Ghaziabad (UP) India. Abstract: Abfraction is the loss of tooth structure at the cervical region from heavy occlusal forces. It is described as one of the causes of lesions found along the cervical margins of teeth. This article critically reviews the literature in favour and against the theory of abfraction. From the literature there is little direct evidence supporting the theory of abfraction, apart from laboratory studies, to indicate that abfraction exists other than as a hypothetical component of cervical wear. I. Introduction Abfraction is the micro structural loss of tooth substance in areas of stress concentration. This occurs most commonly in the cervical region of teeth, where flexure may lead to a breaking away of the thin layer of enamel rods, as well as micro fracture of cementum and dentin1. Since the publication of one of the text books for dentistry by anatomist and physiologist John Hunter in 1778, the definitions and classifications of the terms “attrition”, “abrasion” and “erosion” have been in a state of confusion. Furthermore, the more recent introduction of the terms “abfraction” to designate stress induced non carious lesions have not resolved this dilemma fully2. -

Classification and Diagnosis of Aggressive Periodontitis
Received: 3 November 2016 Revised: 11 October 2017 Accepted: 21 October 2017 DOI: 10.1002/JPER.16-0712 2017 WORLD WORKSHOP Classification and diagnosis of aggressive periodontitis Daniel H. Fine1 Amey G. Patil1 Bruno G. Loos2 1 Department of Oral Biology, Rutgers School Abstract of Dental Medicine, Rutgers University - Newark, NJ, USA Objective: Since the initial description of aggressive periodontitis (AgP) in the early 2Department of Periodontology, Academic 1900s, classification of this disease has been in flux. The goal of this manuscript is to Center of Dentistry Amsterdam (ACTA), review the existing literature and to revisit definitions and diagnostic criteria for AgP. University of Amsterdam and Vrije Universiteit, Amsterdam, The Netherlands Study analysis: An extensive literature search was performed that included databases Correspondence from PubMed, Medline, Cochrane, Scopus and Web of Science. Of 4930 articles Dr. Daniel H. Fine, Department of Oral reviewed, 4737 were eliminated. Criteria for elimination included; age > 30 years old, Biology, Rutgers School of Dental Medicine, Rutgers University - Newark, NJ. abstracts, review articles, absence of controls, fewer than; a) 200 subjects for genetic Email: fi[email protected] studies, and b) 20 subjects for other studies. Studies satisfying the entrance criteria The proceedings of the workshop were were included in tables developed for AgP (localized and generalized), in areas related jointly and simultaneously published in the Journal of Periodontology and Journal of to epidemiology, microbial, host and genetic analyses. The highest rank was given to Clinical Periodontology. studies that were; a) case controlled or cohort, b) assessed at more than one time-point, c) assessed for more than one factor (microbial or host), and at multiple sites. -
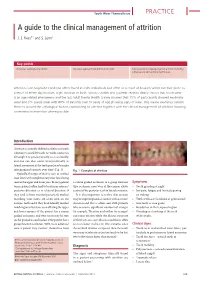
A Guide to the Clinical Management of Attrition
Tooth Wear Themed Issue PRACTICE A guide to the clinical management of attrition J. S. Rees*1 and S. Somi2 Key points Discusses aetiology of attrition. Discusses signs and symptoms of attrition. Discusses clinical management of attrition including adhesive and conventional techniques. Attrition is an enigmatic condition often found in older individuals and often as a result of bruxism which can take place as a result of either day bruxism, night bruxism or both. Various studies and systemic reviews clearly shown that tooth wear is an age-related phenomena and the last Adult Dental Health Survey showed that 15% of participants showed moderate wear and 3% severe wear with 80% of patients over 50 years of age showing signs of wear. This review examines current theories around the aetiological factors contributing to attrition together with the clinical management of attrition focusing on minimal intervention where possible. Introduction Attrition is formally defined as the loss of tooth substance caused by tooth-to-tooth contact so although it is predominantly seen occlusally, attrition can also occur interproximally as lateral movement of the teeth produces broader 1 interproximal contacts over time (Fig. 1). Fig. 1 Examples of attrition Typically, this type of wear is seen as marked wear facets with complimentary wear facets being seen in the upper and lower jaws. In very general a canine guided occlusion to a group function Symptoms terms, patients often tend to brux in an anterior/ type occlusion, once wear of the canines allows • Tooth grinding at night posterior direction or in a lateral direction. If contact of the posterior teeth in lateral excursion. -

Staging and Grading Periodontitis
Staging and Grading Periodontitis The 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions resulted in a new classification of periodontitis characterized by a multidimensional staging and grading system. The charts below provide an overview. Please visit perio.org/2017wwdc for the complete suite of reviews, case definition papers, and consensus reports. PERIODONTITIS: STAGING Staging intends to classify the severity and extent of a patient’s disease based on the measurable amount of destroyed and/or damaged tissue as a result of periodontitis and to assess the specific factors that may attribute to the complexity of long-term case management. Initial stage should be determined using clinical attachment loss (CAL). If CAL is not available, radiographic bone loss (RBL) should be used. Tooth loss due to periodontitis may modify stage definition. One or more complexity factors may shift the stage to a higher level. Seeperio.org/2017wwdc for additional information. Periodontitis Stage I Stage II Stage III Stage IV Interdental CAL 1 – 2 mm 3 – 4 mm ≥5 mm ≥5 mm (at site of greatest loss) Severity Coronal third Coronal third Extending to middle Extending to middle RBL (<15%) (15% - 33%) third of root and beyond third of root and beyond Tooth loss No tooth loss ≤4 teeth ≥5 teeth (due to periodontitis) Local • Max. probing depth • Max. probing depth In addition to In addition to ≤4 mm ≤5 mm Stage II complexity: Stage III complexity: • Mostly horizontal • Mostly horizontal • Probing depths • Need for