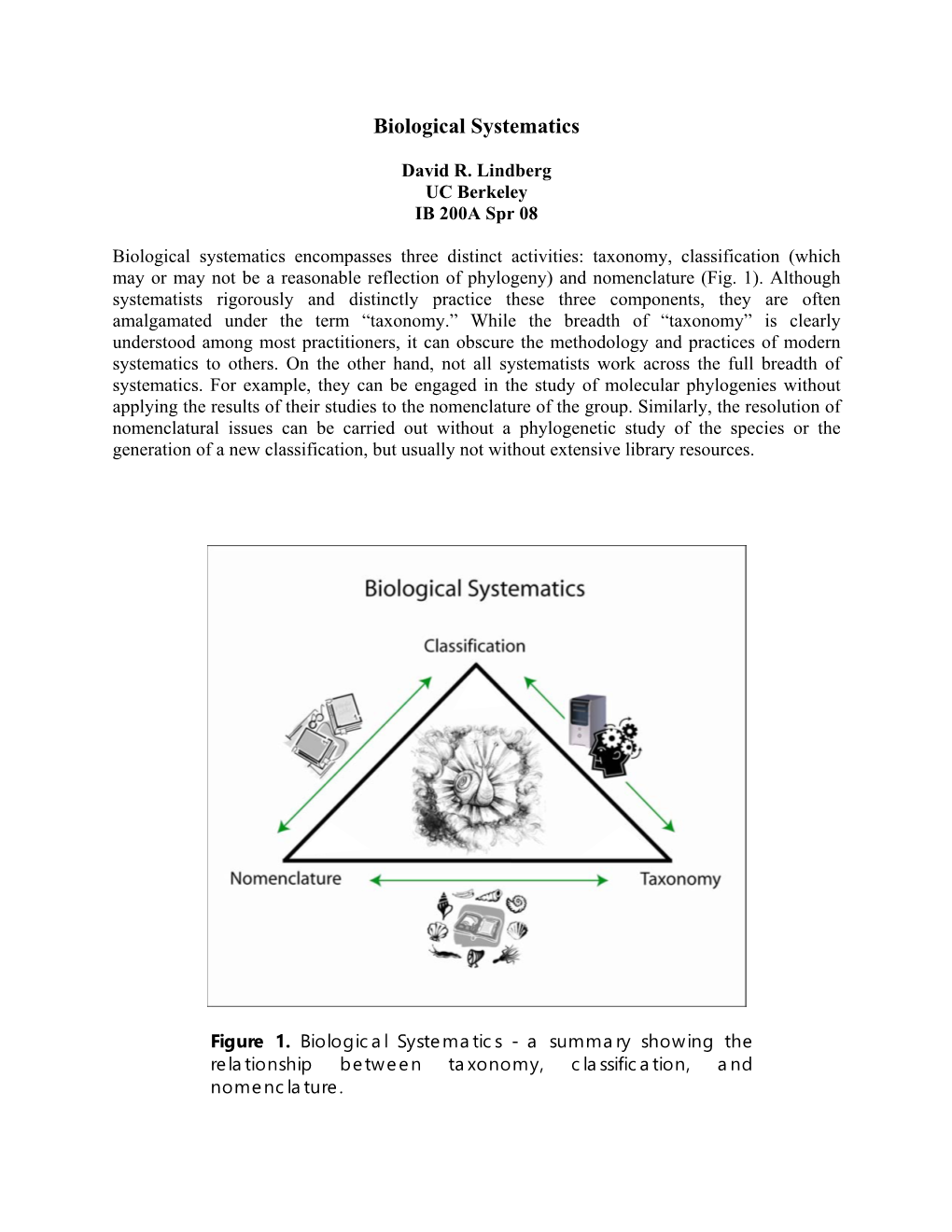
Biological Systematics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Burmese Amber Taxa
Burmese (Myanmar) amber taxa, on-line supplement v.2021.1 Andrew J. Ross 21/06/2021 Principal Curator of Palaeobiology Department of Natural Sciences National Museums Scotland Chambers St. Edinburgh EH1 1JF E-mail: [email protected] Dr Andrew Ross | National Museums Scotland (nms.ac.uk) This taxonomic list is a supplement to Ross (2021) and follows the same format. It includes taxa described or recorded from the beginning of January 2021 up to the end of May 2021, plus 3 species that were named in 2020 which were missed. Please note that only higher taxa that include new taxa or changed/corrected records are listed below. The list is until the end of May, however some papers published in June are listed in the ‘in press’ section at the end, but taxa from these are not yet included in the checklist. As per the previous on-line checklists, in the bibliography page numbers have been added (in blue) to those papers that were published on-line previously without page numbers. New additions or changes to the previously published list and supplements are marked in blue, corrections are marked in red. In Ross (2021) new species of spider from Wunderlich & Müller (2020) were listed as being authored by both authors because there was no indication next to the new name to indicate otherwise, however in the introduction it was indicated that the author of the new taxa was Wunderlich only. Where there have been subsequent taxonomic changes to any of these species the authorship has been corrected below. -

Ediacaran Algal Cysts from the Doushantuo Formation, South China
Geological Magazine Ediacaran algal cysts from the Doushantuo www.cambridge.org/geo Formation, South China Małgorzata Moczydłowska1 and Pengju Liu2 1 Original Article Uppsala University, Department of Earth Sciences, Palaeobiology, Villavägen 16, SE 752 36 Uppsala, Sweden and 2Institute of Geology, Chinese Academy of Geological Science, Beijing 100037, China Cite this article: Moczydłowska M and Liu P. Ediacaran algal cysts from the Doushantuo Abstract Formation, South China. Geological Magazine https://doi.org/10.1017/S0016756820001405 Early-middle Ediacaran organic-walled microfossils from the Doushantuo Formation studied in several sections in the Yangtze Gorges area, South China, show ornamented cyst-like vesicles Received: 24 February 2020 of very high diversity. These microfossils are diagenetically permineralized and observed in pet- Revised: 1 December 2020 rographic thin-sections of chert nodules. Exquisitely preserved specimens belonging to seven Accepted: 2 December 2020 species of Appendisphaera, Mengeosphaera, Tanarium, Urasphaera and Tianzhushania contain Keywords: either single or multiple spheroidal internal bodies inside the vesicles. These structures indicate organic-walled microfossils; zygotic cysts; reproductive stages, endocyst and dividing cells, respectively, and are preserved at early to late Chloroplastida; microalgae; animal embryos; ontogenetic stages in the same taxa. This new evidence supports the algal affiliations for the eukaryotic evolution studied taxa and refutes previous suggestions of Tianzhushania being animal embryo or holo- Author for correspondence: Małgorzata zoan. The first record of a late developmental stage of a completely preserved specimen of Moczydłowska, Email: [email protected] T. spinosa observed in thin-section demonstrates the interior of vesicles with clusters of iden- tical cells but without any cavity that is diagnostic for recognizing algal cysts vs animal diapause cysts. -

With Two New Genera in Burmese Amber G.O
Бiологiчний вiсник МДПУ імені Богдана Хмельницького 6 (3), стор. 157¢164, 2016 Biological Bulletin of Bogdan Chmelnitskiy Melitopol State Pedagogical University, 6 (3), pp. 157¢164, 2016 ARTICLE UDC 595.768 A NEW WEEVIL TRIBE, MEKORHAMPHINI TRIB. NOV. (COLEOPTERA, ITHYCERIDAE) WITH TWO NEW GENERA IN BURMESE AMBER G.O. Poinar, Jr.1, A.E. Brown2, A.A. Legalov3 1Department of Integrative Biology, Oregon State University, Corvallis OR 97331 USA. E-mail: [email protected]. 22629 Euclid Avenue, Berkeley CA 94708 USA. 3Institute of Systematics and Ecology of Animals, Siberian Branch of Russian Academy of Sciences, Frunze str. 11, Novosibirsk 630091 Russia. E-mail: [email protected] A new tribe, Mekorhamphini trib. n., two new genera Mekorhamphus gen. n. and Habropezus gen. n. and two new species (M. gyralommus sp. n. and H. plaisiommus sp. n.) are described from Burmese amber. The new tribe resembles the tribe Mesophyletini but differs from the latter by possessing contiguous procoxal cavities and very wide elytra with regular striae. From the tribe Anchineini, it differs by the contiguous procoxal cavities, precoxal portion of the prosternum elongated, and swollen trochanters. The new taxa can be distinguished from modern Carini by having antennae attached near the middle of the rostrum, an elongated precoxal portion of the prosternum and enlarged trochanters. Key words: Curculionoidea, Carinae, Mekorhamphini trib. n., Mekorhamphus gyralommus gen. et sp. n., Habropezus plaisiommus gen. et sp. n., Early Cretaceous, Cenomanian. Citation: Poinar, G.O., Jr., Brown, A.E., Legalov, A.A. (2016). A new weevil tribe, Mekorhamphini trib. nov. (Coleoptera, Ithyceridae) with two new genera in Burmese amber. -

Systematics, Genetics and Speciation
Systematics, Genetics and Speciation Fundamentals of Fish Biology 27 January 2014 Why should I listen today? Class objectives 1. understand general terms about Systematics of fish 2. be able to list the five methods of categorizing fish groups 3. understand how species evolve via allopatric and sympatric speciation 4. understand the taxonomy and binomial nomenclature behind the system of naming fish Some definitions • Systematics – the study of the evolutionary relationship among organisms • Taxonomy – the science of describing and classifying organisms • Evolutionary Trees – early diagrams used to show relationships among higher levels • Phylogenetic systematics –uses branching diagrams called cladograms – each branch represents a monophyletic group of organisms (e.g. species, families, order…) – uses characteristics that can be quantified and therefore reduces subjective classification Evolutionary Trees Cladograms More definitions • Monophyletic group is a group including an ancestor and all descendants (e.g. vertebrates) • Paraphyletic group is a group containing some but not all descendants of an ancestor (e.g. dinosaurs) • Polyphyly is a group containing descendants of different ancestors (e.g. invertebrates) Other ways of classifying fish • Warm vs cold water fishes (bass and trout) • Saltwater vs freshwater • Pelagic or benthic • Reproductive styles • Trophic level • Freshwater fish based on evolutionary history (primary, secondary, diadromy) Five categories of taxonomic methods • Morphometric measurements • Meristic traits – considered -

Can We Understand Evolution Without Symbiogenesis?
Can We Understand Evolution Without Symbiogenesis? Francisco Carrapiço …symbiosis is more than a mere casual and isolated biological phenomenon: it is in reality the most fundamental and universal order or law of life. Hermann Reinheimer (1915) Abstract This work is a contribution to the literature and knowledge on evolu- tion that takes into account the biological data obtained on symbiosis and sym- biogenesis. Evolution is traditionally considered a gradual process essentially consisting of natural selection, conducted on minimal phenotypical variations that are the result of mutations and genetic recombinations to form new spe- cies. However, the biological world presents and involves symbiotic associations between different organisms to form consortia, a new structural life dimension and a symbiont-induced speciation. The acknowledgment of this reality implies a new understanding of the natural world, in which symbiogenesis plays an important role as an evolutive mechanism. Within this understanding, symbiosis is the key to the acquisition of new genomes and new metabolic capacities, driving living forms’ evolution and the establishment of biodiversity and complexity on Earth. This chapter provides information on some of the key figures and their major works on symbiosis and symbiogenesis and reinforces the importance of these concepts in our understanding of the natural world and the role they play in the establishing of the evolutionary complexity of living systems. In this context, the concept of the symbiogenic superorganism is also discussed. Keywords Evolution · Symbiogenesis · Symbiosis · Symbiogenic superorgan- ism · New paradigm F. Carrapiço (*) Centre for Ecology Evolution and Environmental Change (CE3C); Centre for Philosophy of Science, Department of Plant Biology, Faculty of science, University of Lisbon, Lisbon, Portugal e-mail: [email protected] © Springer International Publishing Switzerland 2015 81 N. -

Plant Systematics: an Overview
I Systematics 1 Plant Systematics: An Overview PLANTS . 3 Evolution . 10 What Is a Plant? . 3 Taxonomy . 10 Plants and the Evolution of Life . 3 Phylogeny . 13 Land Plants . 5 Why Study Systematics? . 13 Why Study Plants? . 6 REVIEW QUESTIONS . 15 SYSTEMATICS . 7 EXERCISES . 16 What Is Systematics? . 7 REFERENCES FOR FURTHER STUDY . 16 This book is about a fascinating fi eld of biology called plant defi ned by the common (but independently evolved) characteristic systematics. The purpose of this chapter is to introduce the of photosynthesis. However, delimiting organismal groups based basics: what a plant is, what systematics is, and the reasons on evolutionary history has gained almost universal acceptance. for studying plant systematics. This latter type of classifi cation directly refl ects the patterns of that evolutionary history and can be used to explicitly test evolutionary hypotheses (discussed later; see Chapter 2). PLANTS An understanding of what plants are requires an explanation of the evolution of life in general. WHAT IS A PLANT? This question can be answered in either of two conceptual PLANTS AND THE EVOLUTION OF LIFE ways. One way, the traditional way, is to defi ne groups of Life is currently classifi ed as three major groups (sometimes organisms such as plants by the characteristics they possess. called domains) of organisms: Archaea (also called Archae- Thus, historically, “plants” included those organisms that pos- bacteria), Bacteria (also called Eubacteria), and Eukarya or sess photosynthesis, cell walls, spores, and a more or less sed- eukaryotes (also spelled eucaryotes). The evolutionary relation- entary behavior. This traditional grouping of plants contained ships of these groups are summarized in the simplifi ed evolu- a variety of microscopic organisms, all of the “algae,” and tionary tree or cladogram of Figure 1.1. -

Systematics, Evolution and Phylogeny of Annelida – a Morphological Perspective
Memoirs of Museum Victoria 71: 247–269 (2014) Published December 2014 ISSN 1447-2546 (Print) 1447-2554 (On-line) http://museumvictoria.com.au/about/books-and-journals/journals/memoirs-of-museum-victoria/ Systematics, evolution and phylogeny of Annelida – a morphological perspective GÜNTER PURSCHKE1,*, CHRISTOPH BLEIDORN2 AND TORSTEN STRUCK3 1 Zoology and Developmental Biology, Department of Biology and Chemistry, University of Osnabrück, Barbarastr. 11, 49069 Osnabrück, Germany ([email protected]) 2 Molecular Evolution and Animal Systematics, University of Leipzig, Talstr. 33, 04103 Leipzig, Germany (bleidorn@ rz.uni-leipzig.de) 3 Zoological Research Museum Alexander König, Adenauerallee 160, 53113 Bonn, Germany (torsten.struck.zfmk@uni- bonn.de) * To whom correspondence and reprint requests should be addressed. Email: [email protected] Abstract Purschke, G., Bleidorn, C. and Struck, T. 2014. Systematics, evolution and phylogeny of Annelida – a morphological perspective . Memoirs of Museum Victoria 71: 247–269. Annelida, traditionally divided into Polychaeta and Clitellata, is an evolutionary ancient and ecologically important group today usually considered to be monophyletic. However, there is a long debate regarding the in-group relationships as well as the direction of evolutionary changes within the group. This debate is correlated to the extraordinary evolutionary diversity of this group. Although annelids may generally be characterised as organisms with multiple repetitions of identically organised segments and usually bearing certain other characters such as a collagenous cuticle, chitinous chaetae or nuchal organs, none of these are present in every subgroup. This is even true for the annelid key character, segmentation. The first morphology-based cladistic analyses of polychaetes showed Polychaeta and Clitellata as sister groups. -

Systematics - BIO 615
Systematics - BIO 615 Outline - History and introduction to phylogenetic inference 1. Pre Lamarck, Pre Darwin “Classification without phylogeny” 2. Lamarck & Darwin to Hennig (et al.) “Classification with phylogeny but without a reproducible method” 3. Hennig (et al.) to today “Classification with phylogeny & a reproducible method” Biosystematics History alpha phylogenetics Aristotle - Scala Naturae - ladder of perfection with humans taxonomy at top - DIFFICULT mental concept to dislodge! (use of terms like “higher” and “lower” for organisms persist) character identification evolution Linnaeus - perpetuated the ladder-like view of life linear, pre evolution descriptions phylogeny 1758 - Linnaeus grouped all animals into 6 higher taxa: 1. Mammals ( top ) 2. Birds collections classification biogeography 3. Reptiles 4. Fishes 5. Insects Describing taxa = assigning names to groups (populations) 6. Worms ( bottom ) = classification Outline - History and introduction to History phylogenetic inference Lamarck - 1800 - Major impact on Biology: 1. Pre Lamarck, Pre Darwin - First public account of evolution - proposed that modern species had descended from common ancestors over “Classification without phylogeny” immense periods of time - Radical! evolution = descent with modification 2. Lamarck & Darwin to Hennig (et al.) - Began with a ladder-like description… but considered “Classification with phylogeny but Linnaeus’s “worms” to be a chaotic “wastebucket” without a reproducible method” taxon 3. Hennig (et al.) to today - He raided the worm group -

Evolutionary History of Life
Evolutionary history of life The evolutionary history of life on Earth traces the processes by which living and fossil organisms evolved, from the earliest emergence of life to the present. Earth formed about 4.5 billion years (Ga) ago and evidence suggests life emerged prior to 3.7 Ga.[1][2][3] (Although there is some evidence of life as early as 4.1 to 4.28 Ga, it remains controversial due to the possible non- biological formation of the purported fossils.[1][4][5][6][7]) The similarities among all known present-day species indicate that they have diverged through the process of evolution from a common ancestor.[8] Approximately 1 trillion species currently live on Earth[9] of which only 1.75–1.8 million have been named[10][11] and 1.6 million documented in a central database.[12] These currently living species represent less than one percent of all species that have ever lived on earth.[13][14] The earliest evidence of life comes from biogenic carbon signatures[2][3] and stromatolite fossils[15] discovered in 3.7 billion- Life timeline Ice Ages year-old metasedimentary rocks from western Greenland. In 2015, 0 — Primates Quater nary Flowers ←Earliest apes possible "remains of biotic life" were found in 4.1 billion-year-old P Birds h Mammals [16][17] – Plants Dinosaurs rocks in Western Australia. In March 2017, putative evidence of Karo o a n ← Andean Tetrapoda possibly the oldest forms of life on Earth was reported in the form of -50 0 — e Arthropods Molluscs r ←Cambrian explosion fossilized microorganisms discovered in hydrothermal -

View Preprint
The origin of animals as microbial host volumes in nutrient- limited seas The microbe-stuffed gut, rather than the genome, represents the most dynamic gene reservoir within complex, multicellular metazoa (animals). Microbes are known to confer increased metabolic efficiency, increased nutrient recovery, and tolerance of ocean acidity to basal taxa such as sponges, arguably the extant taxa most comparable to the first metazoan. We hypothesize that metazoan origins may be rooted in the capability to compartmentalize, metabolize, and exchange genetic material with a modulated microbiome. We present evidence that the most parsimonious adaptive response of clonal eukaryotic colonies experiencing oligotrophic (nutrient-limited) conditions that accompanied Neoproterozoic glaciation events, which were broadly contemporaneous with metazoan origins, is to evolve a morphological volume to harbor a densified microbiome. Dense microbial communities housed within a cavity would increase instances of horizontal gene transfer between microorganisms and host, accelerating evolutionary innovation at the genetic and epigenetic levels for the holobiont. The accelerated tempo of genetic exchange would continue until the host’s metabolic and reproductive cells became spatially and temporally segregated from one another, at which point the process is effectively suppressed with the emergence of specialized gut and reproductive tissues. This framework may lead to new, testable hypotheses regarding metazoan evolution on Earth and a more tractable means of estimating the pervasiveness of complex, multicellular animal-like life with convergent morphologies on other planets. PeerJ Preprints | https://doi.org/10.7287/peerj.preprints.27173v1 | CC BY 4.0 Open Access | rec: 5 Sep 2018, publ: 5 Sep 2018 1 The origin of animals as microbial host volumes in 2 nutrient-limited seas 3 4 Zachary R. -

A Molecular Perspective on Systematics, Taxonomy and Classification Amazonian Discus Fishes of the Genus Symphysodon
SAGE-Hindawi Access to Research International Journal of Evolutionary Biology Volume 2011, Article ID 360654, 16 pages doi:10.4061/2011/360654 Research Article A Molecular Perspective on Systematics, Taxonomy and Classification Amazonian Discus Fishes of the Genus Symphysodon Manuella Villar Amado,1, 2 Izeni P. Farias,1 and Tomas Hrbek1, 3 1 Laboratorio´ de Evoluc¸ao˜ e Gen´etica Animal, Departamento de Biologia, Universidade Federal do Amazonas, Avenida Rodrigo Octavio´ Jordao˜ Ramos, 3000, 69077-000 Manaus, AM, Brazil 2 Instituto Federal de Educac¸ao,˜ Ciˆencia e Tecnologia do Esp´ırito Santo, Unidade Vitoria,´ Avenida Vitoria,´ 1729, 29040-780 Vitoria, ES, Brazil 3 Biology Department, University of Puerto Rico—Rio Piedras, 00931 San Juan, PR, Puerto Rico Correspondence should be addressed to Tomas Hrbek, [email protected] Received 21 December 2010; Accepted 2 May 2011 Academic Editor: Martin J. Genner Copyright © 2011 Manuella Villar Amado et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. With the goal of contributing to the taxonomy and systematics of the Neotropical cichlid fishes of the genus Symphysodon, we analyzed 336 individuals from 24 localities throughout the entire distributional range of the genus. We analyzed variation at 13 nuclear microsatellite markers, and subjected the data to Bayesian analysis of genetic structure. The results indicate that Symphysodon is composed of four genetic groups: group PURPLE—phenotype Heckel and abacaxi; group GREEN—phenotype green; group RED—phenotype blue and brown; and group PINK—populations of Xingu´ and Cameta.´ Although the phenotypes blue and brown are predominantly biological group RED, they also have substantial contributions from other biological groups, and the patterns of admixture of the two phenotypes are different. -

Permissiveness in the Learning and Development of Song Syntax in Swamp Sparrows
ANIMAL BEHAVIOUR, 1999, 58, 93–103 Article No. anbe.1999.1140, available online at http://www.idealibrary.com on Permissiveness in the learning and development of song syntax in swamp sparrows JEFFREY PODOS*, STEPHEN NOWICKI† & SUSAN PETERS† *Department of Ecology and Evolutionary Biology, University of Arizona †Evolution, Ecology and Organismal Biology Group, Department of Zoology, Duke University (Received 5 February 1998; initial acceptance 24 February 1999; final acceptance 24 March 1999; MS. number: A8204R) Vocal learning in swamp sparrows, Melospiza georgiana, is subject to a host of sensory and motor limitations. One such limitation is that young swamp sparrows almost invariably crystallize their songs with a simple trilled syntax, irrespective of the syntax of vocal models from which they learn. A striking exception to this pattern was recently identified by Podos (1996, Animal Behaviour, 51, 1061–1070), who found that large-scale organizational changes in vocal syntax, including the production of an intermit- tent or ‘broken’ syntax, were produced when birds faced limits on vocal performance capacities during motor ontogeny. Our goal in the present study was to determine whether song models with broken syntax could serve as suitable training models for young swamp sparrows, and, if so, if broken syntax could be faithfully reproduced. We hand-reared 10 male swamp sparrows and exposed them to control, rapid and broken song models. Control song models were copied with a high degree of accuracy, as in previous studies. Rapid song models were copied with deficiencies that suggested performance limits on vocal production; such deficiencies included the production of songs with broken syntax and the production of songs in which notes were dropped out as songs progressed.