A Pilot Study of Brain Morphometry Following Donepezil
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Connectivity of BA46 Involvement in the Executive Control of Language
Alfredo Ardila, Byron Bernal and Monica Rosselli Psicothema 2016, Vol. 28, No. 1, 26-31 ISSN 0214 - 9915 CODEN PSOTEG Copyright © 2016 Psicothema doi: 10.7334/psicothema2015.174 www.psicothema.com Connectivity of BA46 involvement in the executive control of language Alfredo Ardila1, Byron Bernal2 and Monica Rosselli3 1 Florida International University, 2 Miami Children’s Hospital and 3 Florida Atlantic University Abstract Resumen Background: Understanding the functions of different brain areas has Estudio de la conectividad del AB46 en el control ejecutivo del lenguaje. represented a major endeavor of contemporary neurosciences. Modern Antecedentes: la comprensión de las funciones de diferentes áreas neuroimaging developments suggest cognitive functions are associated cerebrales representa una de las mayores empresas de las neurociencias with networks rather than with specifi c areas. Objectives. The purpose contemporáneas. Los estudios contemporáneos con neuroimágenes of this paper was to analyze the connectivity of Brodmann area (BA) 46 sugieren que las funciones cognitivas se asocian con redes más que con (anterior middle frontal gyrus) in relation to language. Methods: A meta- áreas específi cas. El propósito de este estudio fue analizar la conectividad analysis was conducted to assess the language network in which BA46 is del área de Brodmann 46 (BA46) (circunvolución frontal media anterior) involved. The DataBase of Brainmap was used; 19 papers corresponding con relación al lenguaje. Método: se llevó a cabo un meta-análisis para to 60 experimental conditions with a total of 245 subjects were included. determinar el circuito o red lingüística en la cual participa BA46. Se utilizó Results: Our results suggest the core network of BA46. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Neural Correlates Underlying Change in State Self-Esteem Hiroaki Kawamichi 1,2,3, Sho K
www.nature.com/scientificreports OPEN Neural correlates underlying change in state self-esteem Hiroaki Kawamichi 1,2,3, Sho K. Sugawara2,4,5, Yuki H. Hamano2,5,6, Ryo Kitada 2,7, Eri Nakagawa2, Takanori Kochiyama8 & Norihiro Sadato 2,5 Received: 21 July 2017 State self-esteem, the momentary feeling of self-worth, functions as a sociometer involved in Accepted: 11 January 2018 maintenance of interpersonal relations. How others’ appraisal is subjectively interpreted to change Published: xx xx xxxx state self-esteem is unknown, and the neural underpinnings of this process remain to be elucidated. We hypothesized that changes in state self-esteem are represented by the mentalizing network, which is modulated by interactions with regions involved in the subjective interpretation of others’ appraisal. To test this hypothesis, we conducted task-based and resting-state fMRI. Participants were repeatedly presented with their reputations, and then rated their pleasantness and reported their state self- esteem. To evaluate the individual sensitivity of the change in state self-esteem based on pleasantness (i.e., the subjective interpretation of reputation), we calculated evaluation sensitivity as the rate of change in state self-esteem per unit pleasantness. Evaluation sensitivity varied across participants, and was positively correlated with precuneus activity evoked by reputation rating. Resting-state fMRI revealed that evaluation sensitivity was positively correlated with functional connectivity of the precuneus with areas activated by negative reputation, but negatively correlated with areas activated by positive reputation. Thus, the precuneus, as the part of the mentalizing system, serves as a gateway for translating the subjective interpretation of reputation into state self-esteem. -

Supplementary Tables
Supplementary Tables: ROI Atlas Significant table grey matter Test ROI # Brainetome area beta volume EG pre vs post IT 8 'superior frontal gyrus, part 4 (dorsolateral area 6), right', 0.773 17388 11 'superior frontal gyrus, part 6 (medial area 9), left', 0.793 18630 12 'superior frontal gyrus, part 6 (medial area 9), right', 0.806 24543 17 'middle frontal gyrus, part 2 (inferior frontal junction), left', 0.819 22140 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1.3 10665 67 'paracentral lobule, part 2 (area 4 lower limb), left', 0.86 13662 EG pre vs post ET 20 'middle frontal gyrus, part 3 (area 46), right', 0.934 28188 21 'middle frontal gyrus, part 4 (ventral area 9/46 ), left' 0.812 27864 31 'inferior frontal gyrus, part 2 (inferior frontal sulcus), left', 0.864 11124 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1 10665 50 'orbital gyrus, part 5 (area 13), right', -1.7 22626 67 'paracentral lobule, part 2 (area 4 lower limb), left', 1.1 13662 180 'cingulate gyrus, part 3 (pregenual area 32), right', 0.9 10665 261 'Cerebellar lobule VIIb, vermis', -1.5 729 IG pre vs post IT 16 middle frontal gyrus, part 1 (dorsal area 9/46), right', -0.8 27567 24 'middle frontal gyrus, part 5 (ventrolateral area 8), right', -0.8 22437 40 'inferior frontal gyrus, part 6 (ventral area 44), right', -0.9 8262 54 'precentral gyrus, part 1 (area 4 head and face), right', -0.9 14175 64 'precentral gyrus, part 2 (caudal dorsolateral area 6), left', -1.3 18819 81 'middle temporal gyrus, part 1 (caudal area 21), left', -1.4 14472 -

Shared Neural Resources of Rhythm and Syntax: an ALE Meta-Analysis Matthew Heard A, B and Yune S
bioRxiv preprint doi: https://doi.org/10.1101/822676; this version posted October 29, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Shared neural resources of rhythm and syntax: An ALE Meta-Analysis Matthew Heard a, b and Yune S. Lee a, b, c † a Department of Speech and Hearing Sciences, The Ohio State University b Neuroscience Graduate Program, The Ohio State University c Chronic Brain Injury, Discovery Theme, The Ohio State University, Columbus, OH 43210, USA † Corresponding author. Email address [email protected] Abstract A growing body of evidence has highlighted behavioral connections between musical rhythm and linguistic syntax, suggesting that these may be mediated by common neural resources. Here, we performed a quantitative meta-analysis of neuroimaging studies using activation likelihood estimate (ALE) to localize the shared neural structures engaged in a representative set of musical rhythm (rhythm, beat, and meter) and linguistic syntax (merge movement, and reanalysis). Rhythm engaged a bilateral sensorimotor network throughout the brain consisting of the inferior frontal gyri, supplementary motor area, superior temporal gyri/temporoparietal junction, insula, the intraparietal lobule, and putamen. By contrast, syntax mostly recruited the left sensorimotor network including the inferior frontal gyrus, posterior superior temporal gyrus, premotor cortex, and supplementary motor area. Intersections between rhythm and syntax maps yielded overlapping regions in the left inferior frontal gyrus, left supplementary motor area, and bilateral insula—neural substrates involved in temporal hierarchy processing and predictive coding. Together, this is the first neuroimaging meta-analysis providing detailed anatomical overlap of sensorimotor regions recruited for musical rhythm and linguistic syntax. -

Seed MNI Coordinates Lobe
MNI Coordinates Seed Lobe (Hemisphere) Region BAa X Y Z FP1 -18 62 0 Frontal Lobe (L) Medial Frontal Gyrus 10 FPz 4 62 0 Frontal Lobe (R) Medial Frontal Gyrus 10 FP2 24 60 0 Frontal Lobe (R) Superior Frontal Gyrus 10 AF7 -38 50 0 Frontal Lobe (L) Middle Frontal Gyrus 10 AF3 -30 50 24 Frontal Lobe (L) Superior Frontal Gyrus 9 AFz 4 58 30 Frontal Lobe (R) Medial Frontal Gyrus 9 AF4 36 48 20 Frontal Lobe (R) Middle Frontal Gyrus 10 AF8 42 46 -4 Frontal Lobe (R) Inferior Frontal Gyrus 10 F7 -48 26 -4 Frontal Lobe (L) Inferior Frontal Gyrus 47 F5 -48 28 18 Frontal Lobe (L) Inferior Frontal Gyrus 45 F3 -38 28 38 Frontal Lobe (L) Precentral Gyrus 9 F1 -20 30 50 Frontal Lobe (L) Superior Frontal Gyrus 8 Fz 2 32 54 Frontal Lobe (L) Superior Frontal Gyrus 8 F2 26 32 48 Frontal Lobe (R) Superior Frontal Gyrus 8 F4 42 30 34 Frontal Lobe (R) Precentral Gyrus 9 F6 50 28 14 Frontal Lobe (R) Middle Frontal Gyrus 46 F8 48 24 -8 Frontal Lobe (R) Inferior Frontal Gyrus 47 FT9 -50 -6 -36 Temporal Lobe (L) Inferior Temporal Gyrus 20 FT7 -54 2 -8 Temporal Lobe (L) Superior Temporal Gyrus 22 FC5 -56 4 22 Frontal Lobe (L) Precentral Gyrus 6 FC3 -44 6 48 Frontal Lobe (L) Middle Frontal Gyrus 6 FC1 -22 6 64 Frontal Lobe (L) Middle Frontal Gyrus 6 FCz 4 6 66 Frontal Lobe (R) Medial Frontal Gyrus 6 FC2 28 8 60 Frontal Lobe (R) Sub-Gyral 6 FC4 48 8 42 Frontal Lobe (R) Middle Frontal Gyrus 6 FC6 58 6 16 Frontal Lobe (R) Inferior Frontal Gyrus 44 FT8 54 2 -12 Temporal Lobe (R) Superior Temporal Gyrus 38 FT10 50 -6 -38 Temporal Lobe (R) Inferior Temporal Gyrus 20 T7/T3 -

Cortical Parcellation Protocol
CORTICAL PARCELLATION PROTOCOL APRIL 5, 2010 © 2010 NEUROMORPHOMETRICS, INC. ALL RIGHTS RESERVED. PRINCIPAL AUTHORS: Jason Tourville, Ph.D. Research Assistant Professor Department of Cognitive and Neural Systems Boston University Ruth Carper, Ph.D. Assistant Research Scientist Center for Human Development University of California, San Diego Georges Salamon, M.D. Research Dept., Radiology David Geffen School of Medicine at UCLA WITH CONTRIBUTIONS FROM MANY OTHERS Neuromorphometrics, Inc. 22 Westminster Street Somerville MA, 02144-1630 Phone/Fax (617) 776-7844 neuromorphometrics.com OVERVIEW The cerebral cortex is divided into 49 macro-anatomically defined regions in each hemisphere that are of broad interest to the neuroimaging community. Region of interest (ROI) boundary definitions were derived from a number of cortical labeling methods currently in use. Protocols from the Laboratory of Neuroimaging at UCLA (LONI; Shattuck et al., 2008), the University of Iowa Mental Health Clinical Research Center (IOWA; Crespo-Facorro et al., 2000; Kim et al., 2000), the Center for Morphometric Analysis at Massachusetts General Hospital (MGH-CMA; Caviness et al., 1996), a collaboration between the Freesurfer group at MGH and Boston University School of Medicine (MGH-Desikan; Desikan et al., 2006), and UC San Diego (Carper & Courchesne, 2000; Carper & Courchesne, 2005; Carper et al., 2002) are specifically referenced in the protocol below. Methods developed at Boston University (Tourville & Guenther, 2003), Brigham and Women’s Hospital (McCarley & Shenton, 2008), Stanford (Allan Reiss lab), the University of Maryland (Buchanan et al., 2004), and the University of Toyoma (Zhou et al., 2007) were also consulted. The development of the protocol was also guided by the Ono, Kubik, and Abernathy (1990), Duvernoy (1999), and Mai, Paxinos, and Voss (Mai et al., 2008) neuroanatomical atlases. -

Functional Connectivity of the Precuneus in Unmedicated Patients with Depression
Biological Psychiatry: CNNI Archival Report Functional Connectivity of the Precuneus in Unmedicated Patients With Depression Wei Cheng, Edmund T. Rolls, Jiang Qiu, Deyu Yang, Hongtao Ruan, Dongtao Wei, Libo Zhao, Jie Meng, Peng Xie, and Jianfeng Feng ABSTRACT BACKGROUND: The precuneus has connectivity with brain systems implicated in depression. METHODS: We performed the first fully voxel-level resting-state functional connectivity (FC) neuroimaging analysis of depression of the precuneus, with 282 patients with major depressive disorder and 254 control subjects. RESULTS: In 125 unmedicated patients, voxels in the precuneus had significantly increased FC with the lateral orbitofrontal cortex, a region implicated in nonreward that is thereby implicated in depression. FC was also increased in depression between the precuneus and the dorsolateral prefrontal cortex, temporal cortex, and angular and supramarginal areas. In patients receiving medication, the FC between the lateral orbitofrontal cortex and precuneus was decreased back toward that in the control subjects. In the 254 control subjects, parcellation revealed superior anterior, superior posterior, and inferior subdivisions, with the inferior subdivision having high connectivity with the posterior cingulate cortex, parahippocampal gyrus, angular gyrus, and prefrontal cortex. It was the ventral subdivision of the precuneus that had increased connectivity in depression with the lateral orbitofrontal cortex and adjoining inferior frontal gyrus. CONCLUSIONS: The findings support the theory that the system in the lateral orbitofrontal cortex implicated in the response to nonreceipt of expected rewards has increased effects on areas in which the self is represented, such as the precuneus. This may result in low self-esteem in depression. The increased connectivity of the precuneus with the prefrontal cortex short-term memory system may contribute to the rumination about low self-esteem in depression. -
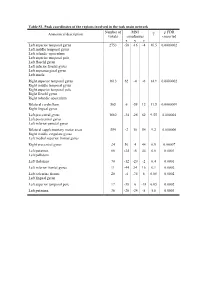
Table S1. Peak Coordinates of the Regions Involved in the Task Main
Table S1. Peak coordinates of the regions involved in the task main network Number of MNI p FDR Anatomical description T voxels coordinates corrected x y z Left superior temporal gyrus 2753 -58 -16 -4 16.5 0.0000002 Left middle temporal gyrus Left rolandic operculum Left superior temporal pole Left Heschl gyrus Left inferior frontal gyrus Left supramarginal gyrus Left insula Right superior temporal gyrus 1613 62 -4 -6 14.9 0.0000002 Right middle temporal gyrus Right superior temporal pole Right Heschl gyrus Right rolandic operculum Bilateral cerebellum 565 6 -58 12 11.5 0.0000009 Right lingual gyrus Left precentral gyrus 1682 -34 -26 62 9.55 0.000004 Left postcentral gyrus Left inferior parietal gyrus Bilateral supplementary motor areas 554 -2 10 54 9.2 0.000006 Right middle cingulate gyrus Left medial superior frontal gyrus Right precentral gyrus 24 56 4 44 6.9 0.00007 Left putamen 66 -24 -6 44 6.6 0.0001 Left pallidum Left thalamus 70 -12 -20 -2 6.4 0.0001 Left inferior frontal gyrus 11 -44 24 16 6.1 0.0002 Left calcarine fissure 20 -4 -74 8 6.08 0.0002 Left lingual gyrus Left superior temporal pole 17 -38 6 -18 6.05 0.0002 Left putamen 36 -28 -24 -8 5.8 0.0003 Table S2. Peak coordinates of the regions showing a main effect of item type Number of MNI p FDR Anatomical description T voxels coordinates corrected x y z Words – Pseudowords Left inferior frontal gyrus 96 -40 24 -6 7.6 0.000001 Left insula Left superior temporal pole Left middle temporal gyrus 141 -56 -36 -2 5.9 0.000015 Left precentral gyrus 26 -50 8 32 4.9 0.00025 Left inferior -

Broca's Region, IBRO History of Neuroscience [ Accessed: Date
Broca’s Region Saturday, December 15 2012, 11:50 AM Broca’s Region Citation: Amunts, K (2007) History of Neuroscience: Broca's Region, IBRO History of Neuroscience [http://www.ibro.info/Pub/Pub_Main_Display.asp?LC_Docs_ID=3489] Accessed: date Katrin Amunts Broca’s region was the first brain region to which a circumscribed function, i.e. language, was linked. Its discovery can be interpreted as the beginning of the scientific theory of the localization of cortical functions. The term originates from anatomo-clinical observations of patients with brain lesions and subsequent disturbances of articulated language, carried out by Pierre Paul Broca (1824-1880) in the middle of the 19th century (Broca, 1861a, b; 1863; 1865, Figure 1). A few years after Broca’s first studies, Wernicke (1848-1904) proposed the first theory of language, which postulated an anterior, motor speech centre (Broca’s region), a posterior, semantic language centre (Wernicke’s region), as well as a fibre tract, the arcuate fasciculus, connecting both regions (Wernicke, 1874). Some decades later, Brodmann’s famous maps (1903a, b; 1906; 1908; 1909; 1910; 1912) proposed that cytoarchitectonically defined cortical areas in the inferior frontal gyrus could be the anatomical correlates of Broca’s region. https://www.ev ernote.com/edit/52741a65-189e-422f -b2da-048e6f 72719b#st=p&n=52741a65-189e-4… 1/13 12/15/12 Ev ernote Web Figure 1: Part of the original publication of Paul Broca, 1861 (M. P. Broca. Remarques sur le siège de la faculté du langage articulé, suivies d'une observation d'aphémie, Perte de la Parole. Bulletins et mémoires de la Société Anatomique de Paris 36:330-357, 1861) in which he reported that the region is located in the posterior third of the left frontal convolution. -
Asymmetry of Amygdala Resting-State Functional Connectivity in Healthy Human Brain
Supplementary Materials Tetereva et al., Asymmetry of Amygdala Resting-State Functional Connectivity in Healthy Human Brain Abbreviations: ACC – anterior cingulate cortex AMYG – amygdala BDI – Beck depression inventory CAUD – caudate nuclei FC – functional connectivity fMRI – functional magnetic resonance imaging HIP – hippocampus, IFGorb – inferior frontal gyrus, pars orbitalis IFGtriang – inferior frontal gyrus, triangular part INS – insula MTG – middle temporal gyrus PCC – posterior cingulate cortex PHG – parahippocampal gyrus preCG – precentral gyrus PTSD – post-traumatic stress disorder ROI – region of interest SFG – superior frontal gyrus, SFGmed – superior frontal gyrus, medial STAI – State-Trait Anxiety Inventory STP – superior temporal pole UNC – uncus vlPFC – ventrolateral prefrontal cortex vmPFC – ventromedial prefrontal cortex. Materials and methods Exclusion criteria for fMRI: We used general exclusion criteria such as prior head trauma, any contraindications against MRI, medication affecting the central nervous system, history of neurologic or psychiatric disorders, consumption of drugs, excessive consumption of alcohol and nicotine, and pregnancy. Preprocessing of MRI data Functional images were realigned with the mean functional image for motion correction. Magnitude and phase values from the field map images served for calculation of the voxel displacement map (VDM) and the subsequent mapping to the mean functional image. Next, each fMRI volume was unwrapped using VDM. After co-registration of the mean functional image with the anatomical image, all functional images were normalized into the standard template of Montreal Neurological Institute (MNI) space with a voxel size of 1.5 x 1.5 x 1.5 mm3 in two stages by the SPM New Segment tool: segmentation of anatomical images into grey and white 1 matter, cerebrospinal fluid, bones, and air, followed by deformation of fMRI volumes and anatomical image using deformation fields. -

Right Inferior Frontal Gyrus Implements Motor Inhibitory Control Via Beta
RESEARCH ARTICLE Right inferior frontal gyrus implements motor inhibitory control via beta-band oscillations in humans Michael Schaum1*, Edoardo Pinzuti1, Alexandra Sebastian2, Klaus Lieb2, Pascal Fries3,4, Arian Mobascher2, Patrick Jung2, Michael Wibral5†, Oliver Tu¨ scher2† 1Systemic Mechanisms of Resilience, Leibniz Institute for Resilience Research, Mainz, Germany; 2Department of Psychiatry and Psychotherapy, University Medical Center of the Johannes Gutenberg University, Mainz, Germany; 3Ernst Stru¨ ngmann Institute (ESI) for Neuroscience in Cooperation with Max Planck Society, Frankfurt, Germany; 4Donders Institute for Brain, Cognition and Behaviour, Radboud University Nijmegen, Nijmegen, Netherlands; 5Campus Institute Dynamics of Biological Networks, Georg-August University, Go¨ ttingen, Germany Abstract Motor inhibitory control implemented as response inhibition is an essential cognitive function required to dynamically adapt to rapidly changing environments. Despite over a decade of research on the neural mechanisms of response inhibition, it remains unclear, how exactly response inhibition is initiated and implemented. Using a multimodal MEG/fMRI approach in 59 subjects, our results reliably reveal that response inhibition is initiated by the right inferior frontal gyrus (rIFG) as a form of attention-independent top-down control that involves the modulation of beta-band activity. Furthermore, stopping performance was predicted by beta-band power, and beta-band connectivity was directed from rIFG to pre-supplementary motor area (pre-SMA), indicating rIFG’s dominance over pre-SMA. Thus, these results strongly support the hypothesis that rIFG initiates *For correspondence: stopping, implemented by beta-band oscillations with potential to open up new ways of spatially [email protected] localized oscillation-based interventions. †These authors contributed equally to this work Competing interests: The authors declare that no Introduction competing interests exist.