Odorant-Evoked Responses of Juxtaglomerular Neurons in the Mammalian Olfactory Bulb
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Cell Migration in the Developing Rodent Olfactory System
Cell. Mol. Life Sci. (2016) 73:2467–2490 DOI 10.1007/s00018-016-2172-7 Cellular and Molecular Life Sciences REVIEW Cell migration in the developing rodent olfactory system 1,2 1 Dhananjay Huilgol • Shubha Tole Received: 16 August 2015 / Revised: 8 February 2016 / Accepted: 1 March 2016 / Published online: 18 March 2016 Ó The Author(s) 2016. This article is published with open access at Springerlink.com Abstract The components of the nervous system are Abbreviations assembled in development by the process of cell migration. AEP Anterior entopeduncular area Although the principles of cell migration are conserved AH Anterior hypothalamic nucleus throughout the brain, different subsystems may predomi- AOB Accessory olfactory bulb nantly utilize specific migratory mechanisms, or may aAOB Anterior division, accessory olfactory bulb display unusual features during migration. Examining these pAOB Posterior division, accessory olfactory bulb subsystems offers not only the potential for insights into AON Anterior olfactory nucleus the development of the system, but may also help in aSVZ Anterior sub-ventricular zone understanding disorders arising from aberrant cell migra- BAOT Bed nucleus of accessory olfactory tract tion. The olfactory system is an ancient sensory circuit that BST Bed nucleus of stria terminalis is essential for the survival and reproduction of a species. BSTL Bed nucleus of stria terminalis, lateral The organization of this circuit displays many evolution- division arily conserved features in vertebrates, including molecular BSTM Bed nucleus of stria terminalis, medial mechanisms and complex migratory pathways. In this division review, we describe the elaborate migrations that populate BSTMa Bed nucleus of stria terminalis, medial each component of the olfactory system in rodents and division, anterior portion compare them with those described in the well-studied BSTMpl Bed nucleus of stria terminalis, medial neocortex. -

Distinct Representations of Olfactory Information in Different Cortical Centres
LETTER doi:10.1038/nature09868 Distinct representations of olfactory information in different cortical centres Dara L. Sosulski1, Maria Lissitsyna Bloom1{, Tyler Cutforth1{, Richard Axel1 & Sandeep Robert Datta1{ Sensory information is transmitted to the brain where it must be behaviours, but is unlikely to specify innate behaviours. Rather, innate processed to translate stimulus features into appropriate beha- olfactory behaviours are likely to result from the activation of genetically vioural output. In the olfactory system, distributed neural activity determined, stereotyped neural circuits. We have therefore developed a in the nose is converted into a segregated map in the olfactory strategy to trace the projections from identified glomeruli in the olfactory bulb1–3. Here we investigate how this ordered representation is bulb to higher olfactory cortical centres. transformed in higher olfactory centres in mice. We have Mitral and tufted cells that innervate a single glomerulus were developed a tracing strategy to define the neural circuits that labelled by electroporation of tetramethylrhodamine (TMR)-dextran convey information from individual glomeruli in the olfactory under the guidance of a two-photon microscope. This technique labels bulb to the piriform cortex and the cortical amygdala. The spatial mitral and tufted cells that innervate a single glomerulus and is suffi- order in the bulb is discarded in the piriform cortex; axons from ciently robust to allow the identification of axon termini within mul- individual glomeruli project diffusely to the piriform without tiple higher order olfactory centres (Figs 1a–c, 2 and Supplementary apparent spatial preference. In the cortical amygdala, we observe Figs 1–4). Labelling of glomeruli in the olfactory bulbs of mice that broad patches of projections that are spatially stereotyped for indi- express GFP under the control of specific odorant receptor promoters vidual glomeruli. -

Comprehensive Connectivity of the Mouse Main Olfactory Bulb: Analysis and Online Digital Atlas
ORIGINAL RESEARCH ARTICLE published: 07 August 2012 NEUROANATOMY doi: 10.3389/fnana.2012.00030 Comprehensive connectivity of the mouse main olfactory bulb: analysis and online digital atlas Houri Hintiryan , Lin Gou , Brian Zingg , Seita Yamashita , Hannah M. Lyden , Monica Y. Song , Arleen K. Grewal , Xinhai Zhang , Arthur W. Toga and Hong-Wei Dong* Laboratory of Neuro Imaging, Department of Neurology, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, USA Edited by: We introduce the first open resource for mouse olfactory connectivity data produced as Jorge A. Larriva-Sahd, Universidad part of the Mouse Connectome Project (MCP) at UCLA. The MCP aims to assemble a Nacional Autónoma de México, whole-brain connectivity atlas for the C57Bl/6J mouse using a double coinjection tracing Mexico method. Each coinjection consists of one anterograde and one retrograde tracer, which Reviewed by: Marco Aurelio M. Freire, Edmond affords the advantage of simultaneously identifying efferent and afferent pathways and and Lily Safra International Institute directly identifying reciprocal connectivity of injection sites. The systematic application for Neurosciences of Natal, Brazil of double coinjections potentially reveals interaction stations between injections and Juan Andrés De Carlos, Instituto allows for the study of connectivity at the network level. To facilitate use of the data, Cajal (Consejo Superior de Investigaciones Científicas), Spain raw images are made publicly accessible through our online interactive visualization *Correspondence: tool, the iConnectome, where users can view and annotate the high-resolution, Hong-Wei Dong, Laboratory of multi-fluorescent connectivity data (www.MouseConnectome.org). Systematic double Neuro Imaging, Department of coinjections were made into different regions of the main olfactory bulb (MOB) and data Neurology, David Geffen School of from 18 MOB cases (∼72 pathways; 36 efferent/36 afferent) currently are available to Medicine, University of California, Los Angeles, 635 Charles E. -
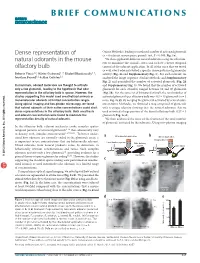
Dense Representation of Natural Odorants in the Mouse Olfactory Bulb
BRIEF COMMUNICATIONS Dense representation of Online Methods), leading to a reduced number of activated glomeruli (n = 6 odorant-mouse pairs, paired t test, P < 0.005; Fig. 1e). natural odorants in the mouse We then applied 40 different natural odorants using the olfactom- eter to minimize the animal’s stress and to have a better temporal olfactory bulb control of the odorant application. In all of the mice that we tested (n = 8), every odorant evoked a specific dense pattern of glomerular Roberto Vincis1,2, Olivier Gschwend1–3, Khaleel Bhaukaurally1–3, activity (Fig. 2a and Supplementary Fig. 1). For each odorant, we Jonathan Beroud1,2 & Alan Carleton1,2 analyzed the image sequence (Online Methods and Supplementary Fig. 2) and quantified the number of activated glomeruli (Fig. 2b In mammals, odorant molecules are thought to activate and Supplementary Fig. 3). We found that the number of activated only a few glomeruli, leading to the hypothesis that odor glomeruli for each stimulus ranged between 10 and 40 glomeruli representation in the olfactory bulb is sparse. However, the (Fig. 2b). For the same set of 30 natural stimuli, the total number of studies supporting this model used anesthetized animals or activated glomeruli per olfactory bulb was 443 ± 15 glomeruli (n = 5 monomolecular odorants at limited concentration ranges. mice; Fig. 2c,d). By merging the glomeruli activated by several odor- Using optical imaging and two-photon microscopy, we found ants (Online Methods), we obtained a map composed of glomeruli that natural odorants at their native concentrations could elicit with a unique identity showing that the natural odorants that we dense representations in the olfactory bulb. -

Functional Organization of Sensory Input to the Olfactory Bulb Glomerulus Analyzed by Two-Photon Calcium Imaging
Functional organization of sensory input to the olfactory bulb glomerulus analyzed by two-photon calcium imaging Matt Wachowiak*, Winfried Denk†, and Rainer W. Friedrich†‡ *Department of Biology, Boston University, 5 Cummington Street, Boston, MA 02215; and †Department of Biomedical Optics, Max Planck Institute for Medical Research, Jahnstrasse 29, D-69120 Heidelberg, Germany Edited by Charles F. Stevens, The Salk Institute for Biological Studies, La Jolla, CA, and approved May 10, 2004 (received for review January 20, 2004) Glomeruli in the olfactory bulb are anatomically discrete modules OSNs distinguished by histochemical markers terminate in dis- receiving input from idiotypic olfactory sensory neurons. To ex- crete subregions of the axonal compartment (36, 37). These data amine the functional organization of sensory inputs to individual raise the possibility that functionally distinct subdivisions exist .glomeruli, we loaded olfactory sensory neurons with a Ca2؉ within a glomerulus indicator and measured odorant-evoked presynaptic Ca2؉ signals Direct measurements of the activity of glomerular afferents within single glomeruli by using two-photon microscopy in anaes- have been difficult because most functional measures of intra- thetized mice. Odorants evoked patterns of discrete Ca2؉ signals glomerular activity lack sufficient spatial resolution or cellular throughout the neuropil of a glomerulus. Across glomeruli, Ca2؉ specificity. Here, we loaded OSNs with a fluorescent Ca2ϩ signals occurred with equal probability in all glomerular regions. indicator and measured patterns of afferent presynaptic activity ؉ Within single glomeruli, the pattern of intraglomerular Ca2 sig- within glomeruli by two-photon Ca2ϩ imaging in anaesthetized nals was indistinguishable for stimuli of different duration, iden- mice. Our data indicate that OSN input to individual glomeruli tity, and concentration. -

Medial Temporal Lobe (The Limbic System)
MEDIAL TEMPORAL LOBE (THE LIMBIC SYSTEM) On the medial surface of the temporal lobe are three structures critical for normal human functioning. From rostral to caudal, they are the olfactory cortex, the amygdala, and the hippocampus. We will look at the anatomy and function of each separately, although they are often grouped together as "the limbic system". A. The olfactory system: The olfactory system actually begins in the roof of the nasal cavity. The olfactory receptors are ciliated epithelial cells with an array of receptors capable of detecting thousands of different odors. However, just as with any sensory system, the receptor neurons themselves do not project to the cerebral hemispheres. Their axons project up through the cribiform plate of the skull to synapse on the dendrites of the mitral cells of the olfactory bulb. The axons of the olfactory receptors make up the elusive cranial nerve I. This fragile tract is susceptible to shearing forces in head trauma, and loss of smell is a surprisingly debilitating injury. Here is an example of a section through olfactory bulb. The olfactory bulb is not a simple relay (something which passively transmits the signal), but is a sophisticated structure in itself. The mitral cell- olfactory neuron synapse is actually within a tangle of axons and dendrites that is called a glomerulus. There is a second cell type tucked around these glomeruli which probably affects how the signal is transmitted. These cells are small and densely packed, which gives them the name "granule cells". However, they bear no relation to the granule cells of the cerebellum or cerebral cortex. -

Taste and Smell Disorders in Clinical Neurology
TASTE AND SMELL DISORDERS IN CLINICAL NEUROLOGY OUTLINE A. Anatomy and Physiology of the Taste and Smell System B. Quantifying Chemosensory Disturbances C. Common Neurological and Medical Disorders causing Primary Smell Impairment with Secondary Loss of Food Flavors a. Post Traumatic Anosmia b. Medications (prescribed & over the counter) c. Alcohol Abuse d. Neurodegenerative Disorders e. Multiple Sclerosis f. Migraine g. Chronic Medical Disorders (liver and kidney disease, thyroid deficiency, Diabetes). D. Common Neurological and Medical Disorders Causing a Primary Taste disorder with usually Normal Olfactory Function. a. Medications (prescribed and over the counter), b. Toxins (smoking and Radiation Treatments) c. Chronic medical Disorders ( Liver and Kidney Disease, Hypothyroidism, GERD, Diabetes,) d. Neurological Disorders( Bell’s Palsy, Stroke, MS,) e. Intubation during an emergency or for general anesthesia. E. Abnormal Smells and Tastes (Dysosmia and Dysgeusia): Diagnosis and Treatment F. Morbidity of Smell and Taste Impairment. G. Treatment of Smell and Taste Impairment (Education, Counseling ,Changes in Food Preparation) H. Role of Smell Testing in the Diagnosis of Neurodegenerative Disorders 1 BACKGROUND Disorders of taste and smell play a very important role in many neurological conditions such as; head trauma, facial and trigeminal nerve impairment, and many neurodegenerative disorders such as Alzheimer’s, Parkinson Disorders, Lewy Body Disease and Frontal Temporal Dementia. Impaired smell and taste impairs quality of life such as loss of food enjoyment, weight loss or weight gain, decreased appetite and safety concerns such as inability to smell smoke, gas, spoiled food and one’s body odor. Dysosmia and Dysgeusia are very unpleasant disorders that often accompany smell and taste impairments. -

Plume Dynamics Structure the Spatiotemporal Activity of Mitral/Tufted Cell Networks in the Mouse Olfactory Bulb
ORIGINAL RESEARCH published: 30 April 2021 doi: 10.3389/fncel.2021.633757 Plume Dynamics Structure the Spatiotemporal Activity of Mitral/Tufted Cell Networks in the Mouse Olfactory Bulb Suzanne M. Lewis 1*, Lai Xu 1, Nicola Rigolli 2,3, Mohammad F. Tariq 4, Lucas M. Suarez 1, Merav Stern 5, Agnese Seminara 3 and David H. Gire 1* 1 Department of Psychology, University of Washington, Seattle, WA, United States, 2 Dipartimento di Fisica, Istituto Nazionale Fisica Nucleare (INFN) Genova, Universitá di Genova, Genova, Italy, 3 CNRS, Institut de Physique de Nice, Université Côte d’Azur, Nice, France, 4 Graduate Program in Neuroscience, University of Washington, Seattle, WA, United States, 5 Department of Applied Mathematics, University of Washington, Seattle, WA, United States Although mice locate resources using turbulent airborne odor plumes, the stochasticity and intermittency of fluctuating plumes create challenges for interpreting odor cues in natural environments. Population activity within the olfactory bulb (OB) is thought to process this complex spatial and temporal information, but how plume dynamics Edited by: impact odor representation in this early stage of the mouse olfactory system is unknown. Shaina M. Short, The University of Utah, United States Limitations in odor detection technology have made it difficult to measure plume Reviewed by: fluctuations while simultaneously recording from the mouse’s brain. Thus, previous Andreas Schaefer, studies have measured OB activity following controlled odor pulses of varying profiles or Francis Crick Institute and University frequencies, but this approach only captures a subset of features found within olfactory College London, London, United Kingdom plumes. Adequately sampling this feature space is difficult given a lack of knowledge Shawn Denver Burton, regarding which features the brain extracts during exposure to natural olfactory scenes. -

Asymmetric Neurotransmitter Release Enables Rapid Odour Lateralization in Drosophila
SUPPLEMENTARY INFORMATION doi:10.1038/nature11747 SUPPLEMENTARY NOTES Note S1: Light offset produced a precise compensatory turn in the direction opposite to the light-evoked turn (Fig. 1g). As a result, the fly’s net lateral displacement after this compensatory turn was close to zero (Fig. 1i). A train of light pulses produced a larger turn, and the fly’s compensatory turn after light offset was commensurately larger, although less precise (Fig. 1i). This suggests that the fly keeps track of how far it has turned away from its original path, and the accuracy of its estimate depends on how far it has strayed. Note S2: Resting GCaMP fluorescence (F) was not significantly different in the right and left glomeruli (p = 0.67, n = 6, paired t-test). This is expected because each glomerulus contains the axons arising from both the right and left antennae. Although one antenna is removed, the cut axons arising from that antenna are still present in the neuropil. Thus, both right and left glomeruli must contain the same number of GCaMP molecules, and so resting fluorescence is the same. Spontaneous ORN activity evidently makes little contribution to F. What is the mechanistic basis for the significant ipsi/contra difference in F/F? A previous study reported that an ORN plasma membrane marker is two-fold more abundant in the ipsilateral glomerulus as compared to the contralateral glomerulus1. This suggests that the ipsilateral arbor is simply larger than the contralateral arbor of the same ORN. If the properties of ipsi- and contralateral branches are the same, then the ipsi/contra asymmetry in F/F should be roughly proportional to the ipsi/contra asymmetry in arbor size. -

Olfactory Bulb Glomeruli: External Tufted Cells Intrinsically Burst at Theta Frequency and Are Entrained by Patterned Olfactory Input
1190 • The Journal of Neuroscience, February 4, 2004 • 24(5):1190–1199 Cellular/Molecular Olfactory Bulb Glomeruli: External Tufted Cells Intrinsically Burst at Theta Frequency and Are Entrained by Patterned Olfactory Input Abdallah Hayar, Sergei Karnup, Michael T. Shipley, and Matthew Ennis Department of Anatomy and Neurobiology, Program in Neuroscience, University of Maryland, Baltimore, Maryland 21201 Glomeruli, the initial sites of synaptic processing in the olfactory system, contain at least three types of neurons collectively referred to as juxtaglomerular (JG) neurons. The role of JG neurons in odor processing is poorly understood. We investigated the morphology, spon- taneous, and sensory-evoked activity of one class of JG neurons, external tufted (ET) cells, using whole-cell patch-clamp and extracellular recordings in rat olfactory bulb slices. ET cells have extensive dendrites that ramify within a single glomerulus or, rarely, in two adjacent glomeruli. All ET neurons exhibit spontaneous rhythmic bursts of action potentials (ϳ1–8 bursts/sec). Bursting is intrinsically gener- ated; bursting persisted and became more regular in the presence of ionotropic glutamate and GABA receptor antagonists. Burst fre- quency is voltage dependent; frequency increased at membrane potentials depolarized relative to rest and decreased during membrane potential hyperpolarization. Spontaneous bursting persisted in blockers of calcium channels that eliminated low-threshold calcium spikes (LTS) in ET cells. ET cells have a persistent sodium current available at membrane potentials that generate spontaneous bursting. Internal perfusion with a fast sodium channel blocker eliminated spontaneous bursting but did not block the LTS. These results suggest that persistent sodium channels are essential for spontaneous burst generation in ET cells. -

Tuning and Topography in an Odor Map on the Rat Olfactory Bulb
The Journal of Neuroscience, February 15, 2001, 21(4):1351–1360 Tuning and Topography in an Odor Map on the Rat Olfactory Bulb Markus Meister1,2 and Tobias Bonhoeffer2 1Harvard University, Cambridge, Massachusetts 02138, and 2Max Planck Institute of Neurobiology, D-82152 Munich- Martinsried, Germany The sense of smell originates in a diverse array of receptor centration dependence and yields the effective affinity with neurons, comprising up to 1000 different types. To understand which a glomerulus responds to an odorant. When tested with how these parallel channels encode chemical stimuli, we re- aliphatic molecules of increasing carbon chain length, many corded the responses of glomeruli in the olfactory bulbs of the glomeruli were sharply tuned for one or two adjacent chain anesthetized rat, by optical imaging of intrinsic signals. Odor lengths. Glomeruli with similar tuning properties were located stimulation produced two kinds of optical responses at the near each other, producing a systematic map of molecular surface of the bulb: a broad diffuse component superposed by chain length on the surface of the olfactory bulb. Given local discrete small spots. Histology showed that the spots corre- inhibitory circuits within the olfactory bulb, this can account for spond to individual glomeruli, and that ϳ400 of them can be the observed functional inhibition between related odors. We monitored in this way. Based on its wavelength-dependence, explore several parallels to the function and architecture of the this optical signal appears to derive from changes in light visual system that help interpret the neural representation of scattering during neural activity. Pure odorants generally acti- odors. -

Phagocytic Glia Are Obligatory Intermediates in Transmission Of
RESEARCH ARTICLE Phagocytic glia are obligatory intermediates in transmission of mutant huntingtin aggregates across neuronal synapses Kirby M Donnelly1, Olivia R DeLorenzo2, Aprem DA Zaya1, Gabrielle E Pisano1, Wint M Thu1, Liqun Luo3,4, Ron R Kopito3, Margaret M Panning Pearce1,2* 1Department of Biological Sciences, University of the Sciences, Philadelphia, United States; 2Program in Neuroscience, University of the Sciences, Philadelphia, United States; 3Department of Biology, Stanford University, Stanford, United States; 4Howard Hughes Medical Institute, Stanford University, Stanford, United States Abstract Emerging evidence supports the hypothesis that pathogenic protein aggregates associated with neurodegenerative diseases spread from cell to cell through the brain in a manner akin to infectious prions. Here, we show that mutant huntingtin (mHtt) aggregates associated with Huntington disease transfer anterogradely from presynaptic to postsynaptic neurons in the adult Drosophila olfactory system. Trans-synaptic transmission of mHtt aggregates is inversely correlated with neuronal activity and blocked by inhibiting caspases in presynaptic neurons, implicating synaptic dysfunction and cell death in aggregate spreading. Remarkably, mHtt aggregate transmission across synapses requires the glial scavenger receptor Draper and involves a transient visit to the glial cytoplasm, indicating that phagocytic glia act as obligatory intermediates in aggregate spreading between synaptically-connected neurons. These findings expand our understanding