Processing Underpinnings of Executive Functions Across Adulthood: the Interplay of Cognitive Control and Inhibition
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

List of Psychological Tests Material Was Prepared for Use As an Aid in Handling Requests for Psychological Testing
List of Psychological Tests Material was prepared for use as an aid in handling requests for psychological testing. The minutes allocated for each test include administration, scoring and write up. Determination of the medical necessity of psychological tests always requires consideration of the clinical facts of the specific case to assure that tests given are a cost-effective means of determining the appropriate treatment for the individual patient and are related to the diagnosis and treatment of covered mental health conditions. INSTRUMENT TYPE AGE MINUT COMMENTS ES 16 Personality Factor Questionnaire (16-PF) Personality 16+ 30 35-60 min per Tests in Print for admin time only ABEL Screen Sexual Interest Adol + 120 Primarily forensic in nature: may not be covered Achenbach System of Empirically Based Assessment 60 Preschool Module Behav Rating Scale 1.5-5 10 Caregiver-Teacher Report Form Behav Rating Scale 1.5-5 10 Child Behavior Checklist (CBCL) Behav Rating Scale 1.5-5 15 Teacher Report Form Behav Rating Scale 6-18 20 Youth Self-Report (YSR) Behav Rating Scale 11-18 20 ACTeERS-ADD-H Comprehensive, Teachers Rating Scale Behav Rating Scale 5 – 13 15 Adaptive Behavior Assessment System (ABAS II) Behav Rating Scale 0-89 30 Adaptive Behavior Scale (ABS) Developmental 3-18 30 ADHD Rating Scale Behav Rating Scale 4 – 18 15 Adolescent Anger Rating Scale Behav Rating Scale 11-19 15 Adolescent Apperception Cards Projective Personality 12 – 19 60 Adult Behavior Checklist (ABCL) Behav Rating Scale 18-89 30 Admin. Time 20 min. + 10 min. for scoring, interpretation, write up. Adolescent Psychopathology Scale Personality Child-adult 60 Alzheimer’s Quick Test (AQT) Neuro Adult 10 Amen System Checklist Behav Rating Scale Adult 15 Animal Naming Neuro Child-adult 10 Aphasia Screening Test (Reitan Indiana) Neuro 5+ 30 Asperger’s Syndrome Diagnostic Scales (ASDS) Rating scale 5-18 20 Attention Deficit Disorder Eval. -

Redalyc.Symptom Validity Assessment in European Countries: Development and State of The
Clínica y Salud ISSN: 1130-5274 [email protected] Colegio Oficial de Psicólogos de Madrid España Merten, Thomas; Dandachi-FitzGerald, Brechje; Hall, Vicki; Schmand, Ben A.; Santamaría, Pablo; González-Ordi, Héctor Symptom validity assessment in European countries: Development and state of the art Clínica y Salud, vol. 24, núm. 3, noviembre, 2013, pp. 129-138 Colegio Oficial de Psicólogos de Madrid Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=180628831001 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Clínica y Salud 24 (2013) 129-138 Vol. 24, No. 3, Noviembre 2013 ISSN: 1130-5274 Clínica y Salud Investigación Empírica en Psicología Número monográfico Evaluación de la simulación y la validez de los síntomas en el ámbito clínico Special Issue Symptom Validity Assessment and Malingering in Clinical Contexts Director/Editor Héctor González Ordi Clínica y Salud Directores Asociados/Associate Editors Mª Isabel Casado Morales Mª Xesús Froján Parga Mª Eugenia Olivares Crespo Miguel Ángel Pérez Nieto Viente Prieto Cabras Mª Fe Rodríguez Muñoz Pablo Santamaría Fernández Albert Sesé Abad www.elsevier.es/clysa Clinical and Health Journal of Empirical Research in Psychology Symptom validity assessment in European countries: Development and state of the art Thomas Mertena*, Brechje Dandachi-FitzGeraldb, -

Comparing Methods to Evaluate Cognitive Deficits in Commercial Airline Pilots
Comparing Methods to Evaluate Cognitive Deficits in Commercial Airline Pilots Joshua Potocko, MD/MPH UCSF Occupational and Environmental Medicine Residency 30 Min Background Philosophy Scope Comparisons Evidence Review: Example Questions for the Group BACKGROUND LCDR Joshua R. Potocko, MC (FS/FMF), USN “The views expressed in this presentation reflect the results of research conducted by the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.” Potocko’s 6 Proclamations: Certain baseline cognitive functions decline with age. Within an individual, these declines are difficult to predict. Between individuals, different types of decline are variable. Understanding the following are critically important to aviation safety: Age-related declines Temporary disturbances in cognitive function, Stable (or progressive) baseline disturbances due to injury, illness, disease, medication, and substance use. When does cognitive dysfunction become unsafe? Cognitive Function Typical, Normal, Adequate, Average Cognitive Inefficiency Circadian, Fatigue, Mood, Stress Cognitive Deficiency Injury, Illness, Meds, Substances Cognitive Disability Above plus regulatory decisions => requires safety factor Baseline New Baseline Unfit Period Injury Illness Meds Baseline Progressive Decline Age XX? Permanently Unfit Disease? PHILOSOPHY Research Question: What is the “best” way to evaluate cognitive deficits in airline pilots? Best: historical? expert opinion?...or -

The Neuropsychological Profile of Professional Action Video Game Players
The neuropsychological profile of professional action video game players Julie Justine Benoit1,2, Eugenie Roudaia3, Taylor Johnson4, Trevor Love4 and Jocelyn Faubert2 1 Department of Psychology, Université de Montréal, Montréal, Québec, Canada 2 Faubert Lab, École d'Optométrie, Université de Montréal, Montréal, Québec, Canada 3 Rotman Research Institute, Toronto, Ontario, Canada 4 Infinite Esports and Entertainment, Frisco, TX, United-States ABSTRACT In the past 20 years, there has been growing research interest in the association between video games and cognition. Although many studies have found that video game players are better than non-players in multiple cognitive domains, other studies failed to replicate these results. Until now, the vast majority of studies defined video game players based on the number of hours an individual spent playing video games, with relatively few studies focusing on video game expertise using performance criteria. In the current study, we sought to examine whether individuals who play video games at a professional level in the esports industry differ from amateur video game players in their cognitive and learning abilities. We assessed 14 video game players who play in a competitive league (Professional) and 16 casual video game players (Amateur) on set of standard neuropsychological tests evaluating processing speed, attention, memory, executive functions, and manual dexterity. We also examined participants' ability to improve performance on a dynamic visual attention task that required tracking multiple objects in three-dimensions (3D-MOT) over five sessions. Professional players showed the largest performance advantage relative to Amateur players in a test of visual spatial memory (Spatial Span), with more modest benefits in a test of selective and sustained attention (d2 Test of Attention), and test of auditory working memory (Digit Span). -
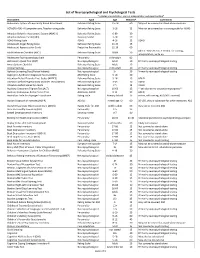
List of Neuropsychological and Psychological Tests.Xlsx
List of Neuropsychological and Psychological Tests *includes administration, scoring, interpretation and report writing* Instrument Type Age Minutes* Comments Achenbach system of Empirically Based Assessment Behavior Rating Scale 1.5‐18 60 May not be covered as stand‐alone measure ACTeERS‐ADD‐H Comprehensive, Teacher rating scale Behavior Rating Scale 3‐18 15 May not be covered as it is rating scale for ADHD Adaptive Behavior Assessment System (ABAS II) Behavior Rating Scale 0‐89 30 Adaptive Behavior Scale (ABS) Developmental 3‐18 30 ADHD Rating Scale ADHD 4‐18 15 ADHD Adolescent Anger Rating Scale Behavior Rating Scale 11‐19 15 Adolescent Apperception Cards Projective Personality 12‐19 60 Admin. Time 20 min. + 10 min. for scoring, Adult Behavior Checklist (ABCL) Behavior Rating Scale 18‐89 30 interpretation, write up Adolescent Psychopathology Scale Personality child‐adult 60 Alzheimer's Quick Test (AQT) Neuropsychological Adult 10 Primarily neuropsychological testing Amen System Checklist Behavior Rating Scale Adult 15 Animal Naming Neuropsychological child‐adult 10 Primarily neuropsychological testing Aphasia Screening Test (Reiten Indiana) Neuropsychological 5+ 30 Primarily neuropsychological testing Asperger's Syndrome Diagnostic Scales (ASDS) ASD Rating Scale 5‐18 20 Attention Deficit Disorder Eval. Scales (ADDES) Behavior Rating Scale 5‐18 15 ADHD Attention Deficit/Hyperactivity Disorder Test (ADHDT) Behavior Rating Scale 3‐23 10 ADHD Attention Deficit Scales for Adults Behavior Rating Scale 17+ 15 ADHD Auditory Consonant Trigram -

Cognitive Performance in an Extreme Case of Anorexia
Hemmingsen et al. BMC Psychiatry (2020) 20:284 https://doi.org/10.1186/s12888-020-02701-1 CASE REPORT Open Access Case report: cognitive performance in an extreme case of anorexia nervosa with a body mass index of 7.7 Simone Daugaard Hemmingsen1,2,3,4,5* , Mia Beck Lichtenstein6,7 , Alia Arif Hussain8,9 , Jan Magnus Sjögren8,9 and René Klinkby Støving1,2,3,4,5 Abstract Background: Studies show that adult patients with anorexia nervosa display cognitive impairments. These impairments may be caused by illness-related circumstances such as low weight. However, the question is whether there is a cognitive adaptation to enduring undernutrition in anorexia nervosa. To our knowledge, cognitive performance has not been assessed previously in a patient with anorexia nervosa with a body mass index as low as 7.7 kg/m2. Case presentation: We present the cognitive profile of a 35-year-old woman with severe and enduring anorexia nervosa who was diagnosed at the age of 10 years. She was assessed with a broad neuropsychological test battery three times during a year. Her body mass index was 8.4, 9.3, and 7.7 kg/m2, respectively. Her general memory performance was above the normal range and she performed well on verbal and design fluency tasks. Her working memory and processing speed were within the normal range. However, her results on cognitive flexibility tasks (set-shifting) were below the normal range. Conclusions: The case study suggests that it is possible to perform normally cognitively despite extreme and chronic malnutrition though set-shifting ability may be affected. -

Do the Stimulant Medications Improve Neuropsychological Performance of College Students with ADHD?
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2010 Do the stimulant medications improve neuropsychological performance of college students with ADHD? Chunqiao Luo Louisiana State University and Agricultural and Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Part of the Psychology Commons Recommended Citation Luo, Chunqiao, "Do the stimulant medications improve neuropsychological performance of college students with ADHD?" (2010). LSU Master's Theses. 3405. https://digitalcommons.lsu.edu/gradschool_theses/3405 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. DO THE STIMULANT MEDICATIONS IMPROVE NEUROPSYCHOLOGICAL PERFORMANCE OF COLLEGE STUDENTS WITH ADHD? A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Master of Arts in The Department of Psychology by Chunqiao Luo B.S., Sun Yat-sen University, 2007 May 2010 TABLE OF CONTENTS ABSTRACT………………………………………………………………………………….iii INTRODUCTION……………………………………………………………………………1 History of ADHD…………………….........................................................................1 Laboratory Evaluation of Symptoms…………………………………………...…...1 Clinical Assessment………………………………………………………………….2 -

Neurocognitive and Psychosocial Outcomes in Patients with Obstructive Sleep Apnea Treated with Continuous Positive Airway Pressure
NEUROCOGNITIVE AND PSYCHOSOCIAL OUTCOMES IN PATIENTS WITH OBSTRUCTIVE SLEEP APNEA TREATED WITH CONTINUOUS POSITIVE AIRWAY PRESSURE by Esther Yuet Ying Lau Submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy at Dalhousie University Halifax, Nova Scotia June 2008 © Copyright by Esther Yuet Ying Lau, 2008 Library and Bibliotheque et 1*1 Archives Canada Archives Canada Published Heritage Direction du Branch Patrimoine de I'edition 395 Wellington Street 395, rue Wellington Ottawa ON K1A0N4 Ottawa ON K1A0N4 Canada Canada Your file Votre reference ISBN: 978-0-494-43943-2 Our file Notre reference ISBN: 978-0-494-43943-2 NOTICE: AVIS: The author has granted a non L'auteur a accorde une licence non exclusive exclusive license allowing Library permettant a la Bibliotheque et Archives and Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par telecommunication ou par I'lnternet, prefer, telecommunication or on the Internet, distribuer et vendre des theses partout dans loan, distribute and sell theses le monde, a des fins commerciales ou autres, worldwide, for commercial or non sur support microforme, papier, electronique commercial purposes, in microform, et/ou autres formats. paper, electronic and/or any other formats. The author retains copyright L'auteur conserve la propriete du droit d'auteur ownership and moral rights in et des droits moraux qui protege cette these. this thesis. Neither the thesis Ni la these ni des extraits substantiels de nor substantial extracts from it celle-ci ne doivent etre imprimes ou autrement may be printed or otherwise reproduits sans son autorisation. -

Seattle Washington, USA Sheraton Seattle Hotel
International Neuropsychological Society 42nd Annual Meeting February 12-15, 2014 Translating Evidence into Practice Seattle Washington, USA Sheraton Seattle Hotel KEYNOTE PRESENTATIONS WEDNESDAY, FEBRUARY 12 Update on Acute Effects and Early A Social Neuroscience Perspective Clinical Trials of Behavioral Recovery After Mild TBI: Lessons on Adolescent Risk-Taking Interventions in Neurologic Patients: Toward the Development of a from Sports Concussion Laurence Steinberg, Ph.D. Developing Evidence Rehabilitation Treatment Taxonomy: Michael McCrea, Ph.D. Chaired by Sureyya Dikmen, Ph.D. A Conceptual Framework John Whyte, M.D., Ph.D. Ecologically Valid Methods of FRIDAY, FEBRUARY 14 Traumatic Brain Injury – Assessment in Neuropsychology. The Challenge to Improve Outcome Why is Autism More Common Chaired by Deirdre Dawson, Ph.D. Jennie Ponsford, Ph.D. THURSDAY, FEBRUARY 13 in Males? Simon Baron-Cohen, Ph.D. Mild Cognitive Best Practices for Enhancing Cognitive Recovery: Restoration, SATURDAY, FEBRUARY 15 Impairment Comes of Age Age-Related Memory Decline: Compensation, and Can We Tell Glenn Smith, Ph.D. New insights from Imaging, Sifting Through the Smoke: the Difference? Genetics, and Biomarkers Uncovering the Impact of Marijuana Featured Debate Hemodynamic Factors Underlying Andrew Saykin, Psy.D. Use on Neurocognition the Pathogenesis and Clinical Chaired by Raul Gonzalez, Ph.D. Expression of Alzheimer’s Disease Angela Jefferson, Ph.D. International Neuropsychological Society 700 Ackerman Road, Suite 625 • Columbus, Ohio 43202 Phone: 614-263-4200 • Fax: 614-263-4366 • Email: [email protected] • www.the-ins.org Performance You Can See & Hear Coming Early Spring Evaluate attention disorders and neurological functioning with the Conners Continuous Performance Tests, now with both visual and auditory attention assessments. -

A Prospective Comparison of Perioperative Embolization and Neuropsychological Outcome in Patients with Carotid Stenosis Treated
A prospective comparison of perioperative embolization and neuropsychological outcome in patients with carotid stenosis treated by carotid endarterectomy versus carotid stenting with distal filter protection or flow reversal Maarten Plessers Doctoral thesis submitted in fulfilment of the requirements to obtain the degree of ‘Doctor in Medical Sciences’ Ghent University 2016 ©2016 Maarten Plessers Universiteit Gent, Belgium This work was supported by a predoctoral Research Grant from the Fund for Scientific Research Flanders (FWO, Belgium) to M.P and by an FWO Research Grant to F.V (3G019009). Alle rechten voorbehouden. Niets uit deze uitgave mag worden verveelvoudigd, opgeslagen in een geautomatiseerd gegevensbestand of openbaar gemaakt, op welke wijze dan ook, zonder de uitdrukkelijke voorafgaande en schriftelijke toestemming van de auteur. All rights preserved. No part of the publication may be reproduced in any form by print, photo print, microfilm, electronic or any other means without written permission from the author. 2 Members of the Doctoral Guidance Committee Promotor Prof. Dr. Guy Vingerhoets Department of Experimental Psychology Faculty of Psychology and Educational Sciences Ghent University Co-promotor Prof. Dr. Frank Vermassen Department of Thoracic and Vascular Surgery Faculty of Medicine and Health Sciences Ghent University Hospital Members of the guidance committee Prof. Dr. Isabelle Van Herzeele Department of Thoracic and Vascular Surgery Faculty of Medicine and Health Sciences Ghent University Hospital Dr. Nathalie Stroobant Members of the Examination Committee Prof. Dr. G.J. de Borst, University Medical Centre Utrecht Prof. Dr. V. De Herdt, Ghent University Prof. Dr. L. Defreyne, Ghent University Prof. Dr. W. Fias, Ghent University Dr. E. Lannoo, Ghent University Hospital Dr. -

National Academy of Neuropsychology Abstracts from the 25Th Annual Meeting, Tampa, Florida October 19–22, 2005 Convention Abst
Archives of Clinical Neuropsychology 20 (2005) 805–950 Abstracts National Academy of Neuropsychology Abstracts from the 25th Annual Meeting, Tampa, Florida October 19–22, 2005 Convention Abstracts Accepted under the Guest Editorship of T. Andrew Zabel (PhD) Kennedy Krieger Institute John Hopkins University School of Medicine Members of the 2005 NAN Poster Program Committee: John Bayless E. Mark Mahone Leigh Beglinger Roy Martin Lynn Blackburn Thomas Martin Jeffrey Browndyke Stephen Moelter Thomas Burns Judith O’Jile Shane Bush Cynthia Salorio Kevin Duff Mark Sandberg David Erlanger Mike Schoenberg Phillip Fastenau Mark Sherer Tania Giovannetti Matthew Thompson Frank Hillary Rodney Vanderploeg Paul Jones Frank Webbe Carrie Kennedy Timothy Wynkoop Gregory Lee Assistant to the Editor Christopher Vaughan 0887-6177/$ – see front matter doi:10.1016/j.acn.2005.06.001 806 Abstracts / Archives of Clinical Neuropsychology 20 (2005) 805–950 ADULT GRAND ROUNDS Moderator: Rick Naugle AGR1 Reversible frontotemporal dementia: verbal fluency content says it all Walker LA, de Meulemeester C A 55-year-old gentleman (M.M.) presented with the cognitive and behavioural profile typically observed in frontotemporal dementia in the context of a diagnosis of spontaneous intracranial hypotension (SIH). Objective: Our goal is to expand upon the only other case report in the literature with this presentation of SIH (Hong et al., 2002), particularly with regard to useful assessment tools, and to illustrate that early diagnosis and treatment can hasten and maximize recovery of cognitive functions. Method: M.M. was healthy until 8 weeks prior to admission to the Neurology in-patient service. Symptoms then included severe orthostatic headache, as well as cognitive and personality changes. -
Clinical Neuropsychology 101 (An Introduction)
Module V. Neuropsychological Assessments "The Impact" Module V - Neurological Assessments Page 2 of 22 Instructions to This Module: - If you have questions we encourage you to talk to your supervisor or you can call the ABI Program Manager under (801) 538-8244. Objectives of This Module: a) Understand what a Neuropsychological Assessment is. b) Understand what indicates a Neuropsychological Assessment. c) Understand what Neuropsychological Assessments measure. d) Understand the tests used in Neuropsychological Assessments. e) Understand the purpose of the tests. Module V - Neurological Assessments Page 3 of 22 Clinical Neuropsychology 101 (An Introduction) The clinical specialty of neuropsychology bridges interest in the biological function of neural cells in the brain, spinal cord, and body with the study of psychological disorders. The neuropsychologist uses objective, scientific techniques to link behaviors to underlying normal and abnormal biological (i.e., brain) processes. The clinical neuropsychologist studies patterns of abnormal behavior to infer the biological abnormalities that might be producing or contributing to those behaviors. Traditionally defined, neuropsychology is the study of (and the assessment, understanding, and modification of) brain-behavior relationships. Neuropsychology seeks to understand how the brain, through structure and neural networks, produces and controls behavior and mental processes, including emotions, personality, thinking, learning and remembering, problem solving, and consciousness. The field is also concerned with how behavior may influence the brain and related physiological processes, as in the emerging field of psychoneuroimmunology (the study that seeks to understand the complex interactions between brain and immune systems, and the implications for physical health). Neuropsychology seeks to gain knowledge about brain and behavior relationships through the study of both healthy and damaged brain systems.