Is Depletion of the Ozone Layer the Principal Cause of Climate Change?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Greenhouse Gas Emissions from Public Lands
The Climate Report 2020: Greenhouse Gas Emissions from Public Lands As the world works to respond to the dire regulations meant to protect the public and the warnings issued last fall by the United Nations environment from these exact decisions. New Environment Programme, the Trump policies have made it cheaper and easier for administration continues to open up as much fossil energy corporations to gain and hold of our shared public lands as possible to fossil control of public lands. And they have hidden fuel extraction.1 And while doing so, the federal from public view the implications of these government continues to keep the public (aka decisions for taxpayers and the planet. the owners of these resources) in the dark on This report seeks to pull the curtain back on the mounting greenhouse gas emissions that this situation and shed light on the range of would result from drilling on our public lands. potential climate consequences of these At the same time, we’ve seen this leasing decisions. administration water down policies and 1 UN. 26 Nov. 2019. stories/press-release/cut-global-emissions-76- https://www.unenvironment.org/news-and- percent-every-year-next-decade-meet-15degc Key Takeaways: The federal government cannot manage development of these leases could be as what it does not measure, yet the Trump high as 6.6 billion MT CO2e. administration is actively seeking to These leasing decisions have significant suppress disclosure of climate emissions and long-term ramifications for our climate from fossil energy leases on public lands. and our ability to stave off the worst The leases issued during the Trump impacts of global warming. -

Aerosol Effective Radiative Forcing in the Online Aerosol Coupled CAS
atmosphere Article Aerosol Effective Radiative Forcing in the Online Aerosol Coupled CAS-FGOALS-f3-L Climate Model Hao Wang 1,2,3, Tie Dai 1,2,* , Min Zhao 1,2,3, Daisuke Goto 4, Qing Bao 1, Toshihiko Takemura 5 , Teruyuki Nakajima 4 and Guangyu Shi 1,2,3 1 State Key Laboratory of Numerical Modeling for Atmospheric Sciences and Geophysical Fluid Dynamics, Institute of Atmospheric Physics, Chinese Academy of Sciences, Beijing 100029, China; [email protected] (H.W.); [email protected] (M.Z.); [email protected] (Q.B.); [email protected] (G.S.) 2 Collaborative Innovation Center on Forecast and Evaluation of Meteorological Disasters/Key Laboratory of Meteorological Disaster of Ministry of Education, Nanjing University of Information Science and Technology, Nanjing 210044, China 3 College of Earth and Planetary Sciences, University of Chinese Academy of Sciences, Beijing 100029, China 4 National Institute for Environmental Studies, Tsukuba 305-8506, Japan; [email protected] (D.G.); [email protected] (T.N.) 5 Research Institute for Applied Mechanics, Kyushu University, Fukuoka 819-0395, Japan; [email protected] * Correspondence: [email protected]; Tel.: +86-10-8299-5452 Received: 21 September 2020; Accepted: 14 October 2020; Published: 17 October 2020 Abstract: The effective radiative forcing (ERF) of anthropogenic aerosol can be more representative of the eventual climate response than other radiative forcing. We incorporate aerosol–cloud interaction into the Chinese Academy of Sciences Flexible Global Ocean–Atmosphere–Land System (CAS-FGOALS-f3-L) by coupling an existing aerosol module named the Spectral Radiation Transport Model for Aerosol Species (SPRINTARS) and quantified the ERF and its primary components (i.e., effective radiative forcing of aerosol-radiation interactions (ERFari) and aerosol-cloud interactions (ERFaci)) based on the protocol of current Coupled Model Intercomparison Project phase 6 (CMIP6). -

Climate Change and Human Health: Risks and Responses
Climate change and human health RISKS AND RESPONSES Editors A.J. McMichael The Australian National University, Canberra, Australia D.H. Campbell-Lendrum London School of Hygiene and Tropical Medicine, London, United Kingdom C.F. Corvalán World Health Organization, Geneva, Switzerland K.L. Ebi World Health Organization Regional Office for Europe, European Centre for Environment and Health, Rome, Italy A.K. Githeko Kenya Medical Research Institute, Kisumu, Kenya J.D. Scheraga US Environmental Protection Agency, Washington, DC, USA A. Woodward University of Otago, Wellington, New Zealand WORLD HEALTH ORGANIZATION GENEVA 2003 WHO Library Cataloguing-in-Publication Data Climate change and human health : risks and responses / editors : A. J. McMichael . [et al.] 1.Climate 2.Greenhouse effect 3.Natural disasters 4.Disease transmission 5.Ultraviolet rays—adverse effects 6.Risk assessment I.McMichael, Anthony J. ISBN 92 4 156248 X (NLM classification: WA 30) ©World Health Organization 2003 All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dis- semination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications—whether for sale or for noncommercial distribution—should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Ozonedisinfection.Pdf
ETI - Environmental Technology Initiative Project funded by the U.S. Environmental Protection Agency under Assistance Agreement No. CX824652 What is disinfection? Human exposure to wastewater discharged into the environment has increased in the last 15 to 20 years with the rise in population and the greater demand for water resources for recreation and other purposes. Disinfection of wastewater is done to prevent infectious diseases from being spread and to ensure that water is safe for human contact and the environment. There is no perfect disinfectant. However, there are certain characteristics to look for when choosing the most suitable disinfectant: • Ability to penetrate and destroy infectious agents under normal operating conditions; • Lack of characteristics that could be harmful to people and the environment; • Safe and easy handling, shipping, and storage; • Absence of toxic residuals, such as cancer-causing compounds, after disinfection; and • Affordable capital and operation and maintenance (O&M) costs. What is ozone disinfection? One common method of disinfecting wastewater is ozonation (also known as ozone disinfection). Ozone is an unstable gas that can destroy bacteria and viruses. It is formed when oxygen molecules (O2) collide with oxygen atoms to produce ozone (O3). Ozone is generated by an electrical discharge through dry air or pure oxygen and is generated onsite because it decomposes to elemental oxygen in a short amount of time. After generation, ozone is fed into a down-flow contact chamber containing the wastewater to be disinfected. From the bottom of the contact chamber, ozone is diffused into fine bubbles that mix with the downward flowing wastewater. See Figure 1 on page 2 for a schematic of the ozonation process. -

Short- and Long-Term Greenhouse Gas and Radiative Forcing Impacts of Changing Water Management in Asian Rice Paddies
Global Change Biology (2004) 10, 1180–1196, doi: 10.1111/j.1365-2486.2004.00798.x Short- and long-term greenhouse gas and radiative forcing impacts of changing water management in Asian rice paddies STEVE FROLKING*,CHANGSHENGLI*, ROB BRASWELL* andJAN FUGLESTVEDTw *Institute for the Study of Earth, Oceans, & Space, 39 College Road, University of New Hampshire, Durham, NH 03824, USA, wCICERO, Center for International Climate and Environmental Research – Oslo, PO Box 1129, Blindern, 0318 Oslo, Norway Abstract Fertilized rice paddy soils emit methane while flooded, emit nitrous oxide during flooding and draining transitions, and can be a source or sink of carbon dioxide. Changing water management of rice paddies can affect net emissions of all three of these greenhouse gases. We used denitrification–decomposition (DNDC), a process-based biogeochemistry model, to evaluate the annual emissions of CH4,N2O, and CO2 for continuously flooded, single-, double-, and triple-cropped rice (three baseline scenarios), and in further simulations, the change in emissions with changing water management to midseason draining of the paddies, and to alternating crops of midseason drained rice and upland crops (two alternatives for each baseline scenario). We used a set of first- order atmospheric models to track the atmospheric burden of each gas over 500 years. We evaluated the dynamics of the radiative forcing due to the changes in emissions of CH4, N2O, and CO2 (alternative minus baseline), and compared these with standard calculations of CO2-equivalent emissions using global warming potentials (GWPs). All alternative scenarios had lower CH4 emissions and higher N2O emissions than their corresponding baseline cases, and all but one sequestered carbon in the soil more slowly. -

ALBEDO ENHANCEMENT by STRATOSPHERIC SULFUR INJECTIONS: a CONTRIBUTION to RESOLVE a POLICY DILEMMA? an Editorial Essay
ALBEDO ENHANCEMENT BY STRATOSPHERIC SULFUR INJECTIONS: A CONTRIBUTION TO RESOLVE A POLICY DILEMMA? An Editorial Essay Fossil fuel burning releases about 25 Pg of CO2 per year into the atmosphere, which leads to global warming (Prentice et al., 2001). However, it also emits 55 Tg S as SO2 per year (Stern, 2005), about half of which is converted to sub-micrometer size sulfate particles, the remainder being dry deposited. Recent research has shown that the warming of earth by the increasing concentrations of CO2 and other greenhouse gases is partially countered by some backscattering to space of solar radiation by the sulfate particles, which act as cloud condensation nuclei and thereby influ- ence the micro-physical and optical properties of clouds, affecting regional precip- itation patterns, and increasing cloud albedo (e.g., Rosenfeld, 2000; Ramanathan et al., 2001; Ramaswamy et al., 2001). Anthropogenically enhanced sulfate particle concentrations thus cool the planet, offsetting an uncertain fraction of the anthro- pogenic increase in greenhouse gas warming. However, this fortunate coincidence is “bought” at a substantial price. According to the World Health Organization, the pollution particles affect health and lead to more than 500,000 premature deaths per year worldwide (Nel, 2005). Through acid precipitation and deposition, SO2 and sulfates also cause various kinds of ecological damage. This creates a dilemma for environmental policy makers, because the required emission reductions of SO2, and also anthropogenic organics (except black carbon), as dictated by health and ecological considerations, add to global warming and associated negative conse- quences, such as sea level rise, caused by the greenhouse gases. -

Global Environmental Issues and Its Remedies
International Journal of Sustainable Energy and Environment Vol. 1, No. 8, September 2013, PP: 120 - 126, ISSN: 2327- 0330 (Online) Available online at www.ijsee.com Research article Global Environmental Issues and its Remedies Dr. MD. Zulfequar Ahmad Khan* Address Present. Permanent Address for Correspondence *Dr. Md Zulfequar Ahmad Khan 21-B, Lane No 3, Associate Professor Jamia Nagar, Zakir Nagar, Department of Geography & Environmental Studies New Delhi-110025 Arba Minch University INDIA Arba Minch, Ethiopia. Mobile No.: +919718502867 Mobile No: +251 923934234 E-mail: [email protected] _____________________________________________________________________________________________ Abstract To the surprise of many out-spoken environmentalists, it, in fact, turns out mankind and technology actually aren’t the only significant causes of global environmental problems. However, before we start to get too comfortable and confidently assume that we as human beings are officially “off the hook,” the fact remains that several “man-made” causes play a significant role in our current, global problems trend. Many human actions affect what people value. One way in which the actions that cause global change are different from most of these is that the effects take decades to centuries to be realized. This fact causes many concerned people to consider taking action now to protect the values of those who might be affected by global environmental change in years to come. But because of uncertainty about how global environmental systems work, and because the people affected will probably live in circumstances very much different from those of today and may have different values, it is hard to know how present-day actions will affect them. -

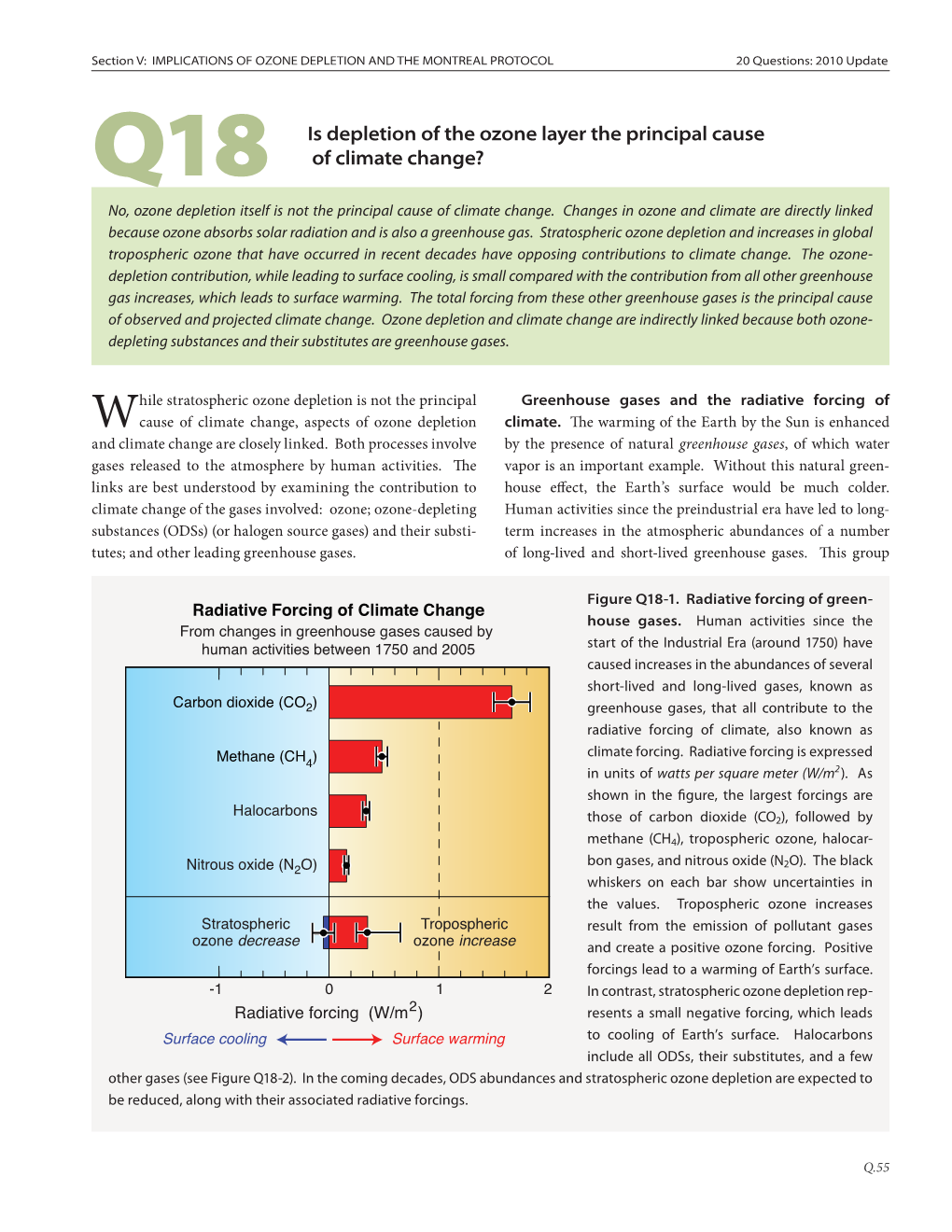
Chapter 1 Ozone and Climate
1 Ozone and Climate: A Review of Interconnections Coordinating Lead Authors John Pyle (UK), Theodore Shepherd (Canada) Lead Authors Gregory Bodeker (New Zealand), Pablo Canziani (Argentina), Martin Dameris (Germany), Piers Forster (UK), Aleksandr Gruzdev (Russia), Rolf Müller (Germany), Nzioka John Muthama (Kenya), Giovanni Pitari (Italy), William Randel (USA) Contributing Authors Vitali Fioletov (Canada), Jens-Uwe Grooß (Germany), Stephen Montzka (USA), Paul Newman (USA), Larry Thomason (USA), Guus Velders (The Netherlands) Review Editors Mack McFarland (USA) IPCC Boek (dik).indb 83 15-08-2005 10:52:13 84 IPCC/TEAP Special Report: Safeguarding the Ozone Layer and the Global Climate System Contents EXECUTIVE SUMMARY 85 1.4 Past and future stratospheric ozone changes (attribution and prediction) 110 1.1 Introduction 87 1.4.1 Current understanding of past ozone 1.1.1 Purpose and scope of this chapter 87 changes 110 1.1.2 Ozone in the atmosphere and its role in 1.4.2 The Montreal Protocol, future ozone climate 87 changes and their links to climate 117 1.1.3 Chapter outline 93 1.5 Climate change from ODSs, their substitutes 1.2 Observed changes in the stratosphere 93 and ozone depletion 120 1.2.1 Observed changes in stratospheric ozone 93 1.5.1 Radiative forcing and climate sensitivity 120 1.2.2 Observed changes in ODSs 96 1.5.2 Direct radiative forcing of ODSs and their 1.2.3 Observed changes in stratospheric aerosols, substitutes 121 water vapour, methane and nitrous oxide 96 1.5.3 Indirect radiative forcing of ODSs 123 1.2.4 Observed temperature -

The Use of Non-CO2 Multipliers for the Climate Impact of Aviation: the Scientific Basis
Workshop on Aviation and Carbon Markets ICAO Headquarters Montreal, Quebec 18-19 June 2008 The use of non-CO2 multipliers for the climate impact of aviation: The scientific basis by Dr. David W. Fahey Earth System Research Laboratory National Oceanic and Atmospheric Administration Boulder, Colorado USA Introduction Outline Aviation and climate change radiative forcings The multiplier concept and limitations Conclusions & recommendations 1 Introduction Aviation contributes to climate change by increasing atmospheric radiative forcing through the emission of gases and aerosols and changing cloud abundance. Radiative forcing is a change in the balance of solar and terrestrial radiation in Earth’s atmosphere. IPCC, AR4 (2007) 2 1 Aviation and climate change Adapted from Wuebbles et al., 2007 3 Aviation and climate change CO2 Non-CO2 The non-CO2 multiplier is an effort to simplify the accounting of aviation climate forcing from effects other than CO2 accumulation. Adapted from Wuebbles et al., 2007 4 2 Global radiative forcing components (1750 - 2005) * Cooling Warming Aviation represents 3% (range 2 - 8%) of anthropogenic radiative forcing in 2005 (includes all components except induced cloudiness) * 5 Adapted from IPCC, AR4 (2007) Global radiative forcing components (1750 - 2005) Aviation RF components Aviation represents 3% (range 2 - 8%) of anthropogenic radiative forcing in 2005 *(includes all components except induced cloudiness) Adapted from IPCC, AR4 (2007) 6 3 Aviation radiative forcing components (1750 - 2005) Cooling Warming Aviation radiative forcing components have been quantified with best estimates except for induced cirrus cloudiness which includes aerosol cloud effects. Radiative forcing is a backward-looking metric that integrates over previous aircraft operations (i.e., 1750-2005) and hence is not a suitable metric for future aviation. -

Ethanol Purification with Ozonation, Activated Carbon Adsorption, and Gas Stripping Shinnosuke Onuki Iowa State University
Agricultural and Biosystems Engineering Agricultural and Biosystems Engineering Publications 9-4-2015 Ethanol purification with ozonation, activated carbon adsorption, and gas stripping Shinnosuke Onuki Iowa State University Jacek A. Koziel Iowa State University, [email protected] William S. Jenks Iowa State University, [email protected] Lingshuang Cai Iowa State University Somchai Rice Iowa State University SeFoe nelloxtw pa thige fors aaddndition addal aitutionhorsal works at: https://lib.dr.iastate.edu/abe_eng_pubs Part of the Agriculture Commons, Bioresource and Agricultural Engineering Commons, and the Organic Chemistry Commons The ompc lete bibliographic information for this item can be found at https://lib.dr.iastate.edu/ abe_eng_pubs/909. For information on how to cite this item, please visit http://lib.dr.iastate.edu/ howtocite.html. This Article is brought to you for free and open access by the Agricultural and Biosystems Engineering at Iowa State University Digital Repository. It has been accepted for inclusion in Agricultural and Biosystems Engineering Publications by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Ethanol purification with ozonation, activated carbon adsorption, and gas stripping Abstract Fermentation of sugar to produce ethanol also produces volatile byproducts. This study was aimed at purifying corn-based ethanol for industrial and pharmaceutical use. The er search was on treatment for 10 impurities removal after distillation. The the anol headspace was sampled with solid-phase microextraction and analyzed with gas chromatography–mass spectrometry. A 40 mg/L ozone treatment resulted in >56% and >36% removal of styrene and 2-pentylfuran, respectively, without significant generation of byproducts. -

Publication No. 17: Ozone Treatment of Private Drinking Water Systems
PRIVATE DRINKING WATER IN CONNECTICUT Publication Date: April 2009 Publication No. 17: Ozone Treatment of Private Drinking Water Systems Effective Against: Pathogenic (disease-causing) organisms including bacteria and viruses, phenols (aromatic organic compounds), some color, taste and odor problems, iron, manganese, and turbidity. Not Effective Against: Large cysts and some other large organisms resulting from possible or probable sewage contamination, inorganic chemicals, and heavy metals. How Ozone (O3) Treatment Works Ozone is a chemical form of pure oxygen. Like chlorine, ozone is a strong oxidizing agent and is used in much the same way to kill disease-causing bacteria and viruses. It is effective against most amoebic cysts, and destroys bacteria and some aromatic organic compounds (such as phenols). Ozone may not kill large cysts and some other large organisms, so these should be eliminated by filtration or other procedures prior to ozone treatment. Ozone is effective in eliminating or controlling color, taste, and odor problems. It also oxidizes iron and manganese. Ozone treatment units are installed as point-of-use treatment systems. Raw water enters one opening and treated water emerges from another. Inside the treatment unit, ozone is produced by an electrical corona discharge or ultraviolet irradiation of dry air or oxygen. The ozone is mixed with the water whenever the water pump is running. Ozone generation units require a system to clean and remove the humidity from the air. For proper disinfection the water to be treated must have negligible color and turbidity levels. The system requires routine maintenance and an ozone treatment system can be very energy consumptive. -

ENERGYENERCY a Balancing Act
Educational Product Educators Grades 9–12 Investigating the Climate System ENERGYENERCY A Balancing Act PROBLEM-BASED CLASSROOM MODULES Responding to National Education Standards in: English Language Arts ◆ Geography ◆ Mathematics Science ◆ Social Studies Investigating the Climate System ENERGYENERGY A Balancing Act Authored by: CONTENTS Eric Barron, College of Earth and Mineral Science, Pennsylvania Grade Levels; Time Required; Objectives; State University, University Park, Disciplines Encompassed; Key Terms; Pennsylvania Prerequisite Knowledge . 2 Prepared by: Scenario. 5 Stacey Rudolph, Senior Science Education Specialist, Institute for Part 1: Understanding the absorption of energy Global Environmental Strategies at the surface of the Earth. (IGES), Arlington, Virginia Question: Does the type of the ground surface John Theon, Former Program influence its temperature? . 5 Scientist for NASA TRMM Part 2: How a change in water phase affects Editorial Assistance, Dan Stillman, surface temperatures. Science Communications Specialist, Institute for Global Environmental Question: How important is the evaporation of Strategies (IGES), Arlington, Virginia water in cooling a surface? . 6 Graphic Design by: Part 3: Determining what controls the temperature Susie Duckworth Graphic Design & of the land surface. Illustration, Falls Church, Virginia Question 1: If my town grows, will it impact the Funded by: area’s temperature? . 7 NASA TRMM Grant #NAG5-9641 Question 2: Why are the summer temperatures in the desert southwest so much higher than at the Give us your feedback: To provide feedback on the modules same latitude in the southeast? . 8 online, go to: Appendix A: Bibliography/Resources . 9 https://ehb2.gsfc.nasa.gov/edcats/ educational_product Appendix B: Answer Keys . 10 and click on “Investigating the Appendix C: National Education Standards.