Sexual Selection and the Evolution of Evolvability
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Biol B242 - Coevolution
BIOL B242 - COEVOLUTION http://www.ucl.ac.uk/~ucbhdjm/courses/b242/Coevol/Coevol.html BIOL B242 - COEVOLUTION So far ... In this course we have mainly discussed evolution within species, and evolution leading to speciation. Evolution by natural selection is caused by the interaction of populations/species with their environments. Today ... However, the environment of a species is always partly biotic. This brings up the possiblity that the "environment" itself may be evolving. Two or more species may in fact coevolve. And coevolution gives rise to some of the most interesting phenomena in nature. What is coevolution? At its most basic, coevolution is defined as evolution in two or more evolutionary entities brought about by reciprocal selective effects between the entities. The term was invented by Paul Ehrlich and Peter Raven in 1964 in a famous article: "Butterflies and plants: a study in coevolution", in which they showed how genera and families of butterflies depended for food on particular phylogenetic groupings of plants. We have already discussed some coevolutionary phenomena: For example, sex and recombination may have evolved because of a coevolutionary arms race between organisms and their parasites; the rate of evolution, and the likelihood of producing resistance to infection (in the hosts) and virulence (in the parasites) is enhanced by sex. We have also discussed sexual selection as a coevolutionary phenomenon between female choice and male secondary sexual traits. In this case, the coevolution is within a single species, but it is a kind of coevolution nonetheless. One of our problem sets involved frequency dependent selection between two types of players in an evolutionary "game". -

Mating Preferences Might Evolve by Natural Selection. If Mating Mate
A GENERAL MODEL OF SEXUAL AND NATURAL SELECTION P. O'DONALD Department of Zoology, University College of North Wales, bangor Received28.xii.66 1.INTRODUCTION FISHERin The Genetical Theory of JVatural Selection (1930) described how mating preferences might evolve by natural selection. If mating behaviour varies among different genotypes, some individuals may have an hereditary disposition to mate with others having particular characteristics. Usually of course it is the females who choose the males and their choice is determined by the likelihood that the males' display will release their mating responses. If some females prefer to mate with those males that have characteristics advantageous in natural selection, then the genotypes that determine such matings will also be selected: the offspring will carry both the advantageous geno- types and the genotypes of the mating preference. Once the mating preference is established, it will itself add to the selective advantage of the preferred genotypes: a "runaway process" as Fisher called it develops. In a paper in Heredity (1963) I described a mathematical model of this type of selection. In the simplest case two loci must be involved: one locus determines the preferred character and the other the mating preference. If there are only two alleles segregating at each locus, ten different genotypes can occur if the loci are linked and nine if they are not. If they are sex-linked, there are i possible genotypes. I derived finite difference equations giving the frequencies of the genotypes in terms of parameters describing the degree of dominance of the preferred genotypes and the recombination fractions of the loci. -

Evolution of Female Choice Under Intralocus Sexual Conflict and Rstb.Royalsocietypublishing.Org Genotype-By-Environment Interactions
Downloaded from http://rstb.royalsocietypublishing.org/ on August 27, 2018 Evolution of female choice under intralocus sexual conflict and rstb.royalsocietypublishing.org genotype-by-environment interactions Xiang-Yi Li1 and Luke Holman2 1Department of Evolutionary Biology and Environmental Studies, University of Zurich, Winterthurerstrasse 190, Research 8057 Zurich, Switzerland 2School of BioSciences, University of Melbourne, Parkville, Victoria 3010, Australia Cite this article: Li X-Y, Holman L. 2018 Evolution of female choice under intralocus X-YL, 0000-0001-8662-0865 sexual conflict and genotype-by-environment In many species, females are hypothesized to obtain ‘good genes’ for their interactions. Phil. Trans. R. Soc. B 373: offspring by mating with males in good condition. However, female pre- 20170425. ferences might deplete genetic variance and make choice redundant. http://dx.doi.org/10.1098/rstb.2017.0425 Additionally, high-condition males sometimes produce low-fitness offspring, for example because of environmental turnover and gene-by-environment interactions (GEIs) for fitness, or because fit males carry sexually antagonistic Accepted: 24 July 2018 alleles causing them to produce unfit daughters. Here, we extend previous theory by investigating the evolution of female mate choice in a spatially One contribution of 14 to a theme issue explicit evolutionary simulation implementing both GEIs and intralocus ‘Linking local adaptation with the evolution of sexual conflict (IASC), under sex-specific hard or soft selection. We show sex differences’. that IASC can weaken female preferences for high-condition males or even cause a preference for males in low condition, depending on the relative benefits of producing well-adapted sons versus daughters, which in turn Subject Areas: depends on the relative hardness of selection on males and females. -

The Effect of Purging on Sexually Selected Traits Through Antagonistic Pleiotropy with Survival Geir H
The effect of purging on sexually selected traits through antagonistic pleiotropy with survival Geir H. Bolstad1, Christophe Pelabon´ 1, Line-K. Larsen2, Ian A. Fleming3, Aslaug˚ Viken2 & Gunilla Rosenqvist1 1Centre for Conservation Biology, Department of Biology, Norwegian University of Science and Technology, 7491 Trondheim, Norway 2Norwegian Biodiversity Information Centre, Erling Skakkes gt. 47, 7491 Trondheim, Norway 3Ocean Sciences Centre, Memorial University of Newfoundland, St. John’s, Newfoundland, Canada A1C 5S7 Keywords Abstract Contextual analysis, genetic architecture, genetic correlation, Poecilia reticulata, Sexuallyselectedtraitsareexpectedtoevolvetoapointwheretheirpositiveeffect sexually selected traits. on reproductive success is counterbalanced by their negative effect on survival. At the genetic level, such a trade-off implies antagonistic pleiotropy between sur- Correspondence vival and the expression of sexually selected traits. Yet, the consequences of such Geir H. Bolstad, Centre for Conservation a genetic architecture have been largely overlooked in studies examining how in- Biology, Department of Biology, Norwegian breeding influences sexually selected traits. These studies have solely interpreted University of Science and Technology, 7491 Trondheim, Norway. Tel: +47 92 03 76 their results as an effect of increased homozygosity. An alternative, however, is 65; Fax: +47 735 96100; that purging of recessive alleles deleterious for survival when inbreeding increases E-mail: [email protected] can negatively affect the expression of sexually selected traits through antagonistic pleiotropy. We tested this hypothesis by analyzing the effects of inbreeding on sev- Received: 15 November 2011; Revised: 24 eral male ornaments and life-history traits across 20 captive populations of guppies February 2012; Accepted: 24 February 2012 (Poecilia reticulata) with varying levels of inbreeding. Only one ornament, orange area, decreased in its expression with an increasing level of inbreeding. -

Mate Choice | Principles of Biology from Nature Education
contents Principles of Biology 171 Mate Choice Reproduction underlies many animal behaviors. The greater sage grouse (Centrocercus urophasianus). Female sage grouse evaluate males as sexual partners on the basis of the feather ornaments and the males' elaborate displays. Stephen J. Krasemann/Science Source. Topics Covered in this Module Mating as a Risky Behavior Major Objectives of this Module Describe factors associated with specific patterns of mating and life history strategies of specific mating patterns. Describe how genetics contributes to behavioral phenotypes such as mating. Describe the selection factors influencing behaviors like mate choice. page 882 of 989 3 pages left in this module contents Principles of Biology 171 Mate Choice Mating as a Risky Behavior Different species have different mating patterns. Different species have evolved a range of mating behaviors that vary in the number of individuals involved and the length of time over which their relationships last. The most open type of relationship is promiscuity, in which all members of a community can mate with each other. Within a promiscuous species, an animal of either gender may mate with any other male or female. No permanent relationships develop between mates, and offspring cannot be certain of the identity of their fathers. Promiscuous behavior is common in bonobos (Pan paniscus), as well as their close relatives, the chimpanzee (P. troglodytes). Bonobos also engage in sexual activity for activities other than reproduction: to greet other members of the community, to release social tensions, and to resolve conflicts. Test Yourself How might promiscuous behavior provide an evolutionary advantage for males? Submit Some animals demonstrate polygamous relationships, in which a single individual of one gender mates with multiple individuals of the opposite gender. -
Towards a Resolution of the Lek Paradox
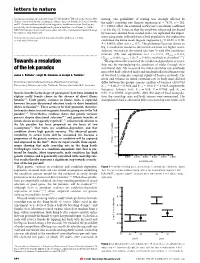
letters to nature discussions regarding experimental design. We also thank G. Taylor from the Oregon State mating. The probability of mating was strongly affected by Climate Center for help with obtaining the climate data. J. A. Pounds, E. Post, M. Vronsky the male's courtship rate (logistic regression x2 79:76, n 232, and N. Chevoteravich provided helpful suggestions on this manuscript. Funding was 1 provided by the Declining Amphibian Population Task Force Seed Grant (to J.M.K.); P , 0.0001; effect size estimated as Pearson's correlation coef®cient NIH/NSF Ecology of Infectious Diseases grant (to J.M.K.); and the Department of Biology, r 0:6; Fig. 1). To ensure that this result was robust and not biased Pennsylvania State University. by measures derived from related males, we replicated the experi- Correspondence and requests for materials should be addressed to J.M.K. ment using males collected from a ®eld population: this replication 2 (e-mail: [email protected]). con®rmed the above result (logistic regression x1 43:30, n 80, P , 0.0001; effect size r 0:7). The preference function shown in Fig. 1 resulted in moderate directional selection for higher court- ship rate (intensity of directional selection (i) with 95% con®dence ................................................................. intervals (CI) and signi®cance test: i 0:331, CIlower 0:121, 22,23 CIupper 0:491; t400 3:20, P 0:0015; methods as described ). Towards a resolution We experimentally examined the condition-dependence of court- ship rate by manipulating the condition of males through their of the lek paradox nutritional state. -

Human Sexual Selection
Available online at www.sciencedirect.com ScienceDirect Human sexual selection David Puts Sexual selection favors traits that aid in competition over Here, I review evidence, focusing on recent findings, mates. Widespread monogamous mating, biparental care, regarding the strength and forms of sexual selection moderate body size sexual dimorphism, and low canine tooth operating over human evolution and consider how sexual dimorphism suggest modest sexual selection operating over selection has shaped human psychology, including psy- human evolution, but other evidence indicates that sexual chological sex differences. selection has actually been comparatively strong. Ancestral men probably competed for mates mainly by excluding The strength of human sexual selection competitors by force or threat, and women probably competed Some evidence suggests that sexual selection has been primarily by attracting mates. These and other forms of sexual relatively weak in humans. Although sexual dimorphisms selection shaped human anatomy and psychology, including in anatomy and behavior may arise from other selective some psychological sex differences. forces, the presence of sexually dimorphic ornamentation, Address weaponry, courtship displays, or intrasexual competition Department of Anthropology and Center for Brain, Behavior and indicates a history of sexual selection [3]. However, men’s Cognition, Pennsylvania State University, University Park, PA 16802, 15–20% greater body mass than women’s is comparable to USA primate species with a modest degree of mating competi- tion among males, and humans lack the canine tooth Corresponding author: Puts, David ([email protected]) dimorphism characteristic of many primates with intense male competition for mates [4]. Moreover, humans exhibit Current Opinion in Psychology 2015, 7:28–32 biparental care and social monogamy, which tend to occur This review comes from a themed issue on Evolutionary psychology in species with low levels of male mating competition [5]. -

Factors Influencing the Diversification of Mating Behavior of Animals
International Journal of Zoology and Animal Biology ISSN: 2639-216X Factors Influencing the Diversification of Mating Behavior of Animals Afzal S1,2*, Shah SS1,2, Afzal T1, Javed RZ1, Batool F1, Salamat S1 and Review Article Raza A1 Volume 2 Issue 2 1Department of zoology, university of Narowal, Pakistan Received Date: January 28, 2019 Published Date: April 24, 2019 2Department of zoology, university of Punjab, Pakistan DOI: 10.23880/izab-16000145 *Corresponding author: Sabila Afzal, Department of zoology, University of Punjab, Pakistan, Email: [email protected] Abstract “Mating system” of a population refers to the general behavioral strategy employed in obtaining mates. In most of them one sex is more philopatric than the other. Reproductive enhancement through increased access to mates or resources and the avoidance of inbreeding are important in promoting sex differences in dispersal. In birds it is usually females which disperse more than males; in mammals it is usually males which disperse more than females. It is argued that the direction of the sex bias is a consequence of the type of mating system. Philopatry will favor the evolution of cooperative traits between members of the sedentary sex. It includes monogamy, Polygyny, polyandry and promiscuity. As an evolutionary strategy, mating systems have some “flexibility”. The existence of extra-pair copulation shows that mating systems identified on the basis of behavioral observations may not accord with actual breeding systems as determined by genetic analysis. Mating systems influence the effectiveness of the contraceptive control of pest animals. This method of control is most effective in monogamous and polygamous species. -

View of Sexual Selection
Evol Biol DOI 10.1007/s11692-015-9359-y ESSAY A Paradox of Genetic Variance in Epigamic Traits: Beyond ‘‘Good Genes’’ View of Sexual Selection 1 2,3 2 Jacek Radwan • Leif Engqvist • Klaus Reinhold Received: 29 June 2015 / Accepted: 4 November 2015 Ó The Author(s) 2015. This article is published with open access at Springerlink.com Abstract Maintenance of genetic variance in secondary Introduction sexual traits, including bizarre ornaments and elaborated courtship displays, is a central problem of sexual selection Darwin (1871) developed the theory of sexual selection to theory. Despite theoretical arguments predicting that strong explain the evolution of traits such as exaggerated antlers sexual selection leads to a depletion of additive genetic or bizarre plumage, which appeared inconsistent with the variance, traits associated with mating success show rela- idea of ‘‘survival of the fittest’’. Darwin argued that sur- tively high heritability. Here we argue that because of vival costs associated with the possession of such traits are trade-offs associated with the production of costly epi- compensated by increased success in reproductive com- gamic traits, sexual selection is likely to lead to an petition, either because these traits serve as weapons in increase, rather than a depletion, of genetic variance in intra-sexual competition, or because they make their those traits. Such trade-offs can also be expected to con- bearers more sexually attractive. tribute to the maintenance of genetic variation in ecologi- Maintenance of genetic variation in sexually selected cally relevant traits with important implications for traits has attracted much attention from evolutionary biol- evolutionary processes, e.g. -

Sexual Selection and Mate Choice
Review TRENDS in Ecology and Evolution Vol.21 No.6 June 2006 Sexual selection and mate choice Malte Andersson1 and Leigh W. Simmons2 1Department of Zoology, University of Gothenburg, SE 405 30 Gothenburg, Sweden 2Centre for Evolutionary Biology, School of Animal Biology (M092), The University of Western Australia, Crawley 6009, WA, Australia The past two decades have seen extensive growth of characterization of genes and their effects, from DNA sexual selection research. Theoretical and empirical sequences via protein to phenotypic expression at the level work has clarified many components of pre- and of the individual, with possible consequences at the postcopulatory sexual selection, such as aggressive population level and above. competition, mate choice, sperm utilization and sexual conflict. Genetic mechanisms of mate choice evolution Evolution of mate choice have been less amenable to empirical testing, but Although mate choice occurs in males and females [4], for molecular genetic analyses can now be used for incisive convenience we refer here to female choice of male traits. experimentation. Here, we highlight some of the As experimental evidence accumulated, mate choice currently debated areas in pre- and postcopulatory became widely recognized, but the genetic mechanisms sexual selection. We identify where new techniques underlying its evolution remain the subject of debate can help estimate the relative roles of the various (Box 1). Showing how mating preferences evolve geneti- selection mechanisms that might work together in the cally is harder than showing that they exist, and the evolution of mating preferences and attractive traits, problem is aggravated by the possibility that several and in sperm–egg interactions. -

Sexual Selection, Speciation and Constraints on Geographical Range Overlap in Birds Christopher Cooney
Washington University in St. Louis Washington University Open Scholarship Biology Faculty Publications & Presentations Biology 5-16-2017 Sexual selection, speciation and constraints on geographical range overlap in birds Christopher Cooney Joseph A. Tobias Jason T. Weir Carlos A. Botero Washington University in St. Louis, [email protected] Nathalie Seddon Follow this and additional works at: https://openscholarship.wustl.edu/bio_facpubs Part of the Behavior and Ethology Commons, Biology Commons, and the Population Biology Commons Recommended Citation Cooney, Christopher; Tobias, Joseph A.; Weir, Jason T.; Botero, Carlos A.; and Seddon, Nathalie, "Sexual selection, speciation and constraints on geographical range overlap in birds" (2017). Biology Faculty Publications & Presentations. 139. https://openscholarship.wustl.edu/bio_facpubs/139 This Article is brought to you for free and open access by the Biology at Washington University Open Scholarship. It has been accepted for inclusion in Biology Faculty Publications & Presentations by an authorized administrator of Washington University Open Scholarship. For more information, please contact [email protected]. 1 Sexual selection, speciation, and constraints on geographical 2 range overlap in birds 3 4 Christopher R. Cooney1,2*, Joseph A. Tobias1,3, Jason T. Weir4, Carlos A. Botero5 & 5 Nathalie Seddon1 6 7 1Edward Grey Institute, Department of Zoology, University of Oxford, South Parks Road, 8 Oxford OX1 3PS, UK. 9 2Department of Animal and Plant Sciences, University of Sheffield, Western Bank, 10 Sheffield S10 2TN, UK. 11 3Department of Life Sciences, Imperial College London, Silwood Park, Buckhurst Road, 12 Ascot, Berkshire, SL5 7PY, UK. 13 4Department Ecology and Evolution and Department of Biological Sciences, University of 14 Toronto Scarborough, Toronto, ON M1C 1A4, Canada. -

Consistent Positive Co-Variation Between Fluctuating Asymmetry and Sexual Trait Size: a Challenge to the Developmental Instability-Sexual Selection Hypothesis
Symmetry 2015, 7, 976-993; doi:10.3390/sym7020976 OPEN ACCESS symmetry ISSN 2073-8994 www.mdpi.com/journal/symmetry Article Consistent Positive Co-Variation between Fluctuating Asymmetry and Sexual Trait Size: A Challenge to the Developmental Instability-Sexual Selection Hypothesis Michal Polak *, Kassie J. Hooker and Frances Tyler Department of Biological Sciences, University of Cincinnati, Cincinnati, OH 45221-0006, USA; E-Mails: [email protected] (K.J.H.); [email protected] (F.T.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-513-556-9736. Academic Editor: John H. Graham Received: 9 March 2015 / Accepted: 1 June 2015 / Published: 3 June 2015 Abstract: The developmental instability (DI)-sexual selection hypothesis proposes that large size and symmetry in secondary sexual traits are favored by sexual selection because they reveal genetic quality. A critical prediction of this hypothesis is that there should exist negative correlations between trait fluctuating asymmetry (FA) and size of condition dependent sexual traits; condition dependent traits should reveal an organism’s overall health and vigor, and be influenced by a multitude of genetic loci. Here, we tested for the predicted negative FA-size correlations in the male sex comb of Drosophila bipectinata. Among field-caught males from five widely separated geographic localities, FA-size correlations were consistently positive, despite evidence that sex comb size is condition dependent. After controlling for trait size, FA was significantly negatively correlated with body size within several populations, indicating that developmental instability in the comb may reveal individual genetic quality. We suggest the possibility that condition dependent traits in some cases tap into independent units of the genome (a restricted set of genes), rather than signaling overall genetic properties of the organism.