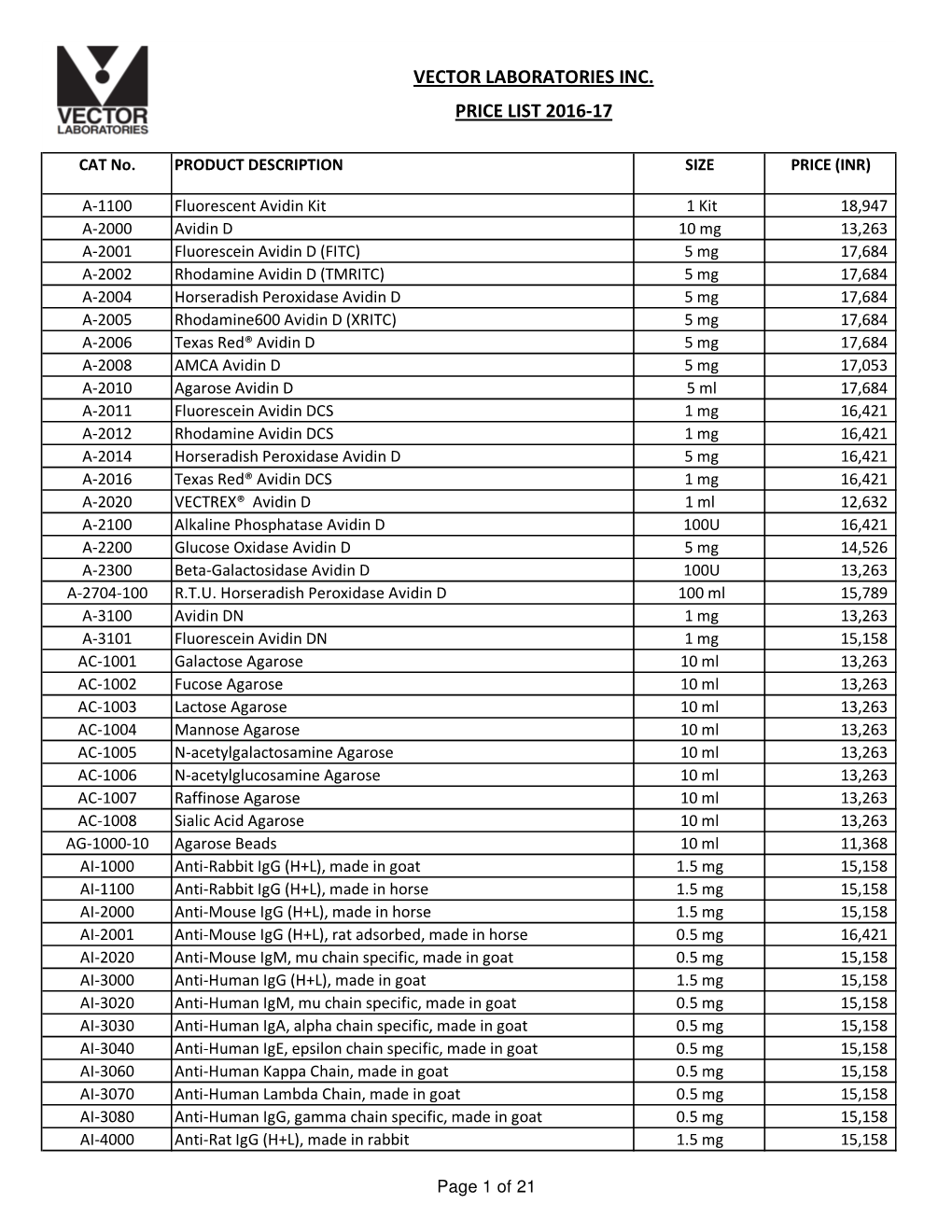
Vector Price List 2016-17
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GLYCO 21 XXI International Symposium on Glycoconjugates
GLYCO 21 XXI International Symposium on Glycoconjugates Abstracts August 21-26, 2011 Vienna, Austria Glycoconj J (2011) 28: 197–36 9 Organising Committee Erika Staudacher (Austria) Leopold März (Austria) Günter Allmaier (Austria) Lothar Brecker (Austria) Josef Glössl (Austria) Hanspeter Kählig (Austria) Paul Kosma (Austria) Lukas Mach (Austria) Paul Messner (Austria) Walther Schmid (Austria) Igor Tvaroška (Slovakia) Reinhard Vlasak (Austria) Iain Wilson (Austria) Scientifi c Program Committee Iain Wilson (Austria) Paul Messner (Austria) Günter Allmaier (Austria) Reginald Bittner (Austria) Paul Kosma (Austria) Eva Stöger (Austria) Graham Warren (Austria) John Hanover (USA; nominated by the Society for Glycobiology) Kelly ten Hagen (USA; nominated by the Society for Glycobiology) supported in abstract selection by Michael Duchêne (Austria) Catherine Merry (UK) Tadashi Suzuki (Japan) Abstracts of the 21st International Symposium on Glycoconjugates The International Glycoconjugate Organisation Gerald W. Hart, President Leopold März, President-elect Paul Gleeson, Immediate Past-president Sandro Sonnino, Secretary Thierry Hennet, Treasurer National Representatives Pedro Bonay (Spain) to replace Angelo Reglero Nicolai Bovin (Russia) Jin Won Cho (Korea) Henrik Clausen (Denmark) Anne Dell (UK) Jukka Finne (Finland) Paul Gleeson (Australia) Jianxin Gu (China) Gerald Hart (USA) Thierry Hennet (Switzerland) Jim Jamieson (Canada) Gordan Lauc (Croatia) Hakon Leffl er (Sweden) Jean-Claude Michalski (France) Werner Reutter (Germany) Sandro Sonnino (Italy) Avadhesha Surolia (India) Ken Kitajima (Japan) Maciej Ugorski (Poland) Johannes F.G. Vliegenthart (The Netherlands) Iain Wilson (Austria) to replace Leopold März Albert M. Wu (Taiwan) Lode Wyns (Belgium) Yehiel Zick (Israel) Glycoconj J (2011) 28: 197–369 Past Presidents Eugene. A. Davidson (USA) Alan B. Foster (UK) Paul Gleeson (Australia) Mary Catherine Glick (USA) Colin Hughes (UK) Roger W. -

Review Sialic Acid-Specific Lectins: Occurrence, Specificity and Function
Cell. Mol. Life Sci. 63 (2006) 1331–1354 1420-682X/06/121331-24 DOI 10.1007/s00018-005-5589-y Cellular and Molecular Life Sciences © Birkhäuser Verlag, Basel, 2006 Review Sialic acid-specific lectins: occurrence, specificity and function F. Lehmanna, *, E. Tiralongob and J. Tiralongoa a Institute for Glycomics, Griffith University (Gold Coast Campus), PMB 50 Gold Coast Mail Centre Australia 9726 (Australia), Fax: +61 7 5552 8098; e-mail: [email protected] b School of Pharmacy, Griffith University (Gold Coast Campus), PMB 50 Gold Coast Mail Centre Australia 9726 (Australia) Received 13 December 2005; received after revision 9 February 2006; accepted 15 February 2006 Online First 5 April 2006 Abstract. Sialic acids consist of a family of acidic nine- through specific interactions with lectins, a family of carbon sugars that are typically located at the terminal po- proteins that recognise and bind sugars. This review will sitions of a variety of glycoconjugates. Naturally occur- present a detailed overview of our current knowledge re- ring sialic acids show an immense diversity of structure, garding the occurrence, specificity and function of sialic and this reflects their involvement in a variety of biologi- acid-specific lectins, particularly those that occur in vi- cally important processes. One such process involves the ruses, bacteria and non-vertebrate eukaryotes. direct participation of sialic acids in recognition events Keywords. Sialic acid, lectin, sialoglycoconjugate, sialic acid-specific lectin, adhesin, infectious disease, immunology. Introduction [1, 2]. The largest structural variations of naturally occurring Sia are at carbon 5, which can be substituted with either an Sialic acids (Sia) are a family of nine-carbon a-keto acids acetamido, hydroxyacetamido or hydroxyl moiety to form (Fig. -

Food Microbiology Characterization of Plant Lectins for Their Ability To
Food Microbiology 82 (2019) 231–239 Contents lists available at ScienceDirect Food Microbiology journal homepage: www.elsevier.com/locate/fm Characterization of plant lectins for their ability to isolate Mycobacterium T avium subsp. paratuberculosis from milk Bernhard F. Hobmaiera, Karina Lutterberga, Kristina J.H. Kleinworta, Ricarda Mayerb, Sieglinde Hirmera, Barbara Amanna, Christina Hölzelb,c, Erwin P. Märtlbauerb, ∗ Cornelia A. Deega, a Chair of Animal Physiology, Department of Veterinary Sciences, LMU Munich, Veterinärstraße 13, D-80539, Munich, Germany b Chair of Hygiene and Technology of Milk, Department of Veterinary Sciences, LMU Munich, Schönleutnerstr 8, D-85764, Oberschleißheim, Germany c Institute of Animal Breeding and Husbandry, Faculty of Agricultural and Nutritional Sciences, CAU Kiel, Hermann-Rodewald-Str. 6, 24098 Kiel, Germany 1. Introduction combined with PCR (phage-PCR). The zoonotic potential of MAP is still under discussion (Kuenstner et al., 2017). It could potentially play a Mycobacterium avium subsp. paratuberculosis is the causative agent of role in inflammatory bowel diseases, like Crohn's disease and ulcerative paratuberculosis or Johne's disease, a chronic granulomatous enteritis colitis (Timms et al., 2016). Additionally, MAP infections could be in- in cattle and small ruminants, causing emaciation, decreased milk volved in the pathogenesis of autoimmune diseases like type 1 diabetes, production and, in cattle, severe diarrhea (Arsenault et al., 2014). After multiple sclerosis, rheumatoid arthritis and Hashimoto's thyroiditis infection, ruminants go through a long, asymptomatic subclinical (Garvey, 2018; Waddell et al., 2015). Although the causal link between phase, in which they cannot reliably be determined by standard diag- MAP and these diseases is not proven, the possible association ne- nostic tests (Li et al., 2017). -

Banlec-Egfp Chimera As a Tool for Evaluation of Lectin Binding to High-Mannose Glycans on Microorganisms
biomolecules Article BanLec-eGFP Chimera as a Tool for Evaluation of Lectin Binding to High-Mannose Glycans on Microorganisms Zorana Lopandi´c 1 , Luka Dragaˇcevi´c 2, Dragan Popovi´c 3 , Uros Andjelkovi´c 3,4, Rajna Mini´c 2 and Marija Gavrovi´c-Jankulovi´c 1,* 1 Department of Biochemistry, Faculty of Chemistry, University of Belgrade, 11000 Belgrade, Serbia; [email protected] 2 Institute of Virology, Vaccines and Sera, 11152 Belgrade, Serbia; [email protected] (L.D.); [email protected] (R.M.) 3 Institute of Chemistry, Technology and Metallurgy, National Institute of the Republic of Serbia, University of Belgrade, 11000 Belgrade, Serbia; [email protected] (D.P.); [email protected] (U.A.) 4 Department of Biotechnology, University of Rijeka, 5100 Rijeka, Croatia * Correspondence: [email protected]; Tel.: +381-11-3336-661 Abstract: Fluorescently labeled lectins are useful tools for in vivo and in vitro studies of the structure and function of tissues and various pathogens such as viruses, bacteria, and fungi. For the evaluation of high-mannose glycans present on various glycoproteins, a three-dimensional (3D) model of the chimera was designed from the crystal structures of recombinant banana lectin (BanLec, Protein Data Bank entry (PDB): 5EXG) and an enhanced green fluorescent protein (eGFP, PDB 4EUL) by applying molecular modeling and molecular mechanics and expressed in Escherichia coli. BanLec- eGFP, produced as a soluble cytosolic protein of about 42 kDa, revealed β-sheets (41%) as the Citation: Lopandi´c,Z.; Dragaˇcevi´c, predominant secondary structures, with the emission peak maximum detected at 509 nm (excitation L.; Popovi´c,D.; Andjelkovi´c,U.; wavelength 488 nm). -

Raybiotech Lectin Array 70 --Detect Glycan Profiles Using 70 Lectins
RayBiotech Lectin Array 70 --Detect glycan profiles using 70 lectins User Manual (Version 2) August 20, 2018 Cat # GA-Lectin-70 RayBiotech, Inc. We Provide You With Excellent Protein Array Systems and Service Tel:(Toll Free) 1-888-494-8555 or 770-729-2992; Fax: 770-206-2393; Website:www.RayBiotech.com Email: [email protected] AAA, AAL, ACG, ACL, ASA, BanLec, BC2L-A, BC2LCN, BPA, Calsepa, CGL2, CNL, Con A, DBA, Discoidin I, Discoidin II, DSA, ECA, EEL, F17AG, Gal1, Gal1-S, Gal2, Gal3, Gal3C-S, Lectins printed on Gal7-S, Gal9, GNA, GRFT, GS-I, GS-II, HHA, Jacalin, LBA, LCA, slides (70) LEA, Lentil, Lotus, LSL-N, MAA, Malectin, MOA, MPL, NPA, Orysata, PA-IIL, PA-IL, PALa, PHA-E, PHA-L, PHA-P, PNA, PPL, PSA, PSL1a, PTL, RS-Fuc, SAMB, SBA, SJA, SNA-I, SNA-II, STL, UDA, UEA-I, UEA-II, VFA, VVA, WFA, WGA One standard glass slide is spotted with 14 wells of Format identical lectin sub-arrays. Each lectin is printed in duplicate on every sub-array Detection Method Fluorescence with laser scanner: Cy3 equivalent dye Sample Volume 50 – 100 l per array Reproducibility CV <20% Assay duration 6 hrs 1 RayBiotech Lectin Array 70 Kit TABLE OF CONTENTS I. Overview………………………………………………………………………………………………………………………………..………………….… 1 Introduction…................................................................................................................................................ 3 How It Works………………………………………………………………………………………………………………………………………... 4 II. Materials Provided……………………………………………………………………………………………….………………..…….. 5 III. General Considerations………………………………………………………………………………………………….….…… 6 A. Label-Based vs. Sandwich-Based Method…………………………………………… 6 B. Preparation of Samples……………………………………………………………………………………………….… 6 C. Handling Glass Slides…………………………………………………………………………………………..……….……. 7 D. Incubation…………………………………………………………………………………………………………………………………….…… 7 IV. Protocol……………………………………………………………………………………………………………………………………………………….… 8 A. Dialysis of Sample…………………………………………………….……………………………………………………………. 8 B. Biotin-labeling Sample………………………………………………………….…………………………………………. 9 C. -

Lectins and Glycobiology State of the Art Labeling & Detection Systems
LECTINS AND GLYCOBIOLOGY STATE OF THE ART LABELING & DETECTION SYSTEMS Vector Laboratories Inc., USA Vector Laboratories Ltd., UK Vector Laboratories, Inc., Canada 30 Ingold Road 3 Accent Park, Bakewell Road 3390 South Service Road Burlingame, CA 94010 Orton Southgate, Peterborough PE2 6XS Burlington, Ontario L7N 3J5 (800) 227-6666 (orders) (01733) 237999 (telephone) (888) 629-2121 (orders only) (650) 697-3600 (technical service) (01733) 237119 (fax) (905) 681-0900 (tel) (650) 697-0339 (fax) [email protected] (email) (905) 681-0166 (fax) [email protected] (email) www.vectorlabs.com [email protected] (email) www.vectorlabs.com www.vectorlabs.com Introduction Since the 1880’s, it has been known that extracts from certain plants could agglutinate red blood cells. In the 1940’s, agglutinins were discovered which could “select” types of cells based on their blood group activities. Although “lectin” was originally coined to define agglutinins that could discriminate among types of red blood cells, today the term is used more generally and includes sugar-binding proteins from many sources regardless of their ability to agglutinate cells. Lectins have been found in plants, viruses, microorganisms, and animals but despite their ubiquity, in many cases their biological function is unclear. Most lectins are multimeric, consisting of non-covalently associated subunits. It is this multimeric structure that gives lectins their ability to agglutinate cells or form precipitates with glycoconjugates in a manner similar to antigen-antibody interactions. This unique group of proteins has provided researchers with powerful tools to explore a myriad of biological structures and processes. Because of the specificity that each lectin has toward a particular carbohydrate structure, even oligosaccharides with identical sugar compositions can be distinguished or separated. -

Complement C3a Signaling Facilitates Skeletal Muscle Regeneration by Regulating Monocyte Function and Trafficking
ARTICLE DOI: 10.1038/s41467-017-01526-z OPEN Complement C3a signaling facilitates skeletal muscle regeneration by regulating monocyte function and trafficking Congcong Zhang1, Chunxiao Wang1, Yulin Li1, Takashi Miwa2, Chang Liu1, Wei Cui1, Wen-Chao Song2 & Jie Du1 Regeneration of skeletal muscle following injury is accompanied by transient inflammation. Here we show that complement is activated in skeletal muscle injury and plays a key role 1234567890 during regeneration. Genetic ablation of complement C3 or its inactivation with Cobra Venom Factor (CVF) result in impaired muscle regeneration following cardiotoxin-induced injury in mice. The effect of complement in muscle regeneration is mediated by the alternative pathway and C3a receptor (C3aR) signaling, as deletion of Cfb, a key alternative pathway component, or C3aR leads to impaired regeneration and reduced monocyte/macrophage infiltration. Monocytes from C3aR-deficient mice express a reduced level of adhesion molecules, cytokines and genes associated with antigen processing and presentation. Exo- genous administration of recombinant CCL5 to C3aR-deficient mice rescues the defects in inflammatory cell recruitment and regeneration. These findings reveal an important role of complement C3a in skeletal muscle regeneration, and suggest that manipulating complement system may produce therapeutic benefit in muscle injury and regeneration. 1 Beijing AnZhen Hospital, Capital Medical University, The Key Laboratory of Remodeling-related Cardiovascular Diseases, Ministry of Education, Beijing Institute of Heart, Lung and Blood Vessel Diseases, Beijing 100029, China. 2 Department of Pharmacology and Institute for Translational Medicine and Therapeutics, University of Pennsylvania, Perelman School of Medicine, Philadelphia, PA 19104, USA. Correspondence and requests for materials should be addressed to W.-C.S. -

Immuno-Electron and Confocal Laser Scanning Microscopy of the Glycocalyx
biology Article Immuno-Electron and Confocal Laser Scanning Microscopy of the Glycocalyx Shailey Gale Twamley 1,2, Anke Stach 1, Heike Heilmann 3, Berit Söhl-Kielczynski 4, Verena Stangl 1,2, Antje Ludwig 1,2,*,† and Agnieszka Münster-Wandowski 3,*,† 1 Medizinische Klinik für Kardiologie und Angiologie, Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, 10117 Berlin, Germany; [email protected] (S.G.T.); [email protected] (A.S.); [email protected] (V.S.) 2 DZHK (German Centre for Cardiovascular Research), Partner Site, 10117 Berlin, Germany 3 Institute of Integrative Neuroanatomy, Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, 10117 Berlin, Germany; [email protected] 4 Institute for Integrative Neurophysiology—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, 10117 Berlin, Germany; [email protected] * Correspondence: [email protected] (A.L.); [email protected] (A.M.-W.) † These authors equally contributed. Simple Summary: The glycocalyx (GCX) is a hydrated, gel-like layer of biological macromolecules attached to the cell membrane. The GCX acts as a barrier and regulates the entry of external substances into the cell. The function of the GCX is highly dependent on its structure and composition. Citation: Twamley, S.G.; Stach, A.; Pathogenic factors can affect the protective structure of the GCX. We know very little about the three- Heilmann, H.; Söhl-Kielczynski, B.; dimensional organization of the GXC. The tiny and delicate structures of the GCX are difficult Stangl, V.; Ludwig, A.; to study by microscopic techniques. -

(12) Patent Application Publication (10) Pub. No.: US 2004/0043923 A1 Parma Et Al
US 2004.0043923A1. (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0043923 A1 Parma et al. (43) Pub. Date: Mar. 4, 2004 (54) HIGHAFFINITY NUCLEIC ACID LIGANDS on Jun. 5, 1996, which is a continuation-in-part of TO LECTINS application No. 08/479,724, filed on Jun. 7, 1995, now Pat. No. 5,780.228, and which is a continuation (75) Inventors: David H. Parma, Boulder, CO (US); in-part of application No. 08/472,256, filed on Jun. 7, Brian Hicke, Boulder, CO (US); 1995, now Pat. No. 6,001,988, and which is a con Philippe Bridonneau, Boulder, CO tinuation-in-part of application No. 08/472,255, filed (US); Larry Gold, Boulder, CO (US) on Jun. 7, 1995, now Pat. No. 5,766,853, and which is a continuation-in-part of application No. 08/477, Correspondence Address: 829, filed on Jun. 7, 1995, now abandoned, which is SWANSON & BRATSCHUN L.L.C. a continuation-in-part of application No. 07/714, 131, 1745 S.HEA CENTER DRIVE filed on Jun. 10, 1991, now Pat. No. 5,475,096, which SUTE 330 is a continuation-in-part of application No. 07/536, HIGHLANDS RANCH, CO 80129 (US) 428, filed on Jun. 11, 1990, now abandoned. (73) Assignee: GILEAD SCIENCES, INC. Publication Classification (21) Appl. No.: 10/409,627 (51) Int. Cl." ......................... A61K 48/00; A61K 38/16; C12O 1/68 (22) Filed: Apr. 7, 2003 (52) U.S. Cl. ...................................... 514/8; 435/6; 514/44 Related U.S. Application Data (57) ABSTRACT (60) Continuation of application No. -

Banana Lectin from Musa Paradisiaca Is Mitogenic for Cow and Pig PBMC Via IL-2 Pathway and ELF1
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 2 August 2021 Article Banana lectin from Musa paradisiaca is mitogenic for cow and pig PBMC via IL-2 pathway and ELF1 Roxane L. Degroote 1, Lucia Korbonits 1, Franziska Stetter 1, Kristina J. H. Kleinwort 1, Marie-Christin Schilloks 1, Barbara Amann 1, Sieglinde Hirmer 1, Stefanie M. Hauck 2 and Cornelia A. Deeg 1 * 1 Chair of Animal Physiology, Department of Veterinary Sciences, LMU Munich, D-82152 Martinsried, Germany; [email protected] (R.D.); [email protected] (L.K.); [email protected] (F.S.); [email protected] (K.K.); [email protected] (M.S.); [email protected] (B.A.); [email protected] (S.H.) 2 Research Unit Protein Science, Helmholtz Center Munich, German Research Center for Environmental Health, D-80939 Munich, Germany; [email protected] * Correspondence: [email protected] 1 Abstract: The aim of the study was to gain deeper insights in the potential for polyclonal stimulation of PBMC from cows and pigs with banana lectin (BanLec) from Musa paradisiaca. BanLec induced a marked proliferative response in cow and pig PBMC. The response in pigs was even higher than to Concanavalin A. Molecular processes associated with respective responses were examined with differential proteome analyses. Discovery proteomic experiments was applied to BanLec stimulated PBMC and cellular and secretome responses were analyzed with label free LC-MS/MS. In PBMC, 3955 proteins were identified. After polyclonal stimulation with BanLec, 459 proteins showed significantly changed abundance in PBMC. -

Development of Polymeric Matrices Based on Banana Plant Extracts for Biomedical Applications
Rosa Estela Abreu do Nascimento Bachelor Degree in Biochemistry Development of Polymeric Matrices Based on Banana Plant Extracts for Biomedical Applications Dissertation to obtain the Master Degree in Biochemistry Supervisor: Dr. Luísa Alexandra Graça Neves, Auxiliary Researcher Faculdade de Ciências e Tecnologia da Universidade Nova de Lisboa (FCT NOVA) Jury: President: Dr. Teresa Sacadura Santos-Silva, Assistant Professor, FCT NOVA Examiner: Dr. Cristiana Andreia Vieira Torres, Researcher, FCT NOVA Supervisor: Dr. Luísa Alexandra Graça Neves, Auxiliary Researcher, FCT NOVA November 2019 Mestre em Bioquímica ii Rosa Estela Abreu do Nascimento Bachelor Degree in Biochemistry Development of Polymeric Matrices Based on Banana Plant Extracts for Biomedical Applications Dissertation to obtain the Master Degree in Biochemistry Mestre em Bioquímica Supervisor : Dr. Luísa Alexandra Graça Neves, Auxiliary Researcher Faculdade de Ciências e Tecnologia da Universidade Nova de Lisboa (FCT NOVA) November 2019 iii iv Copyright Development of Polymeric Matrices Based on Banana Plant Extracts for Biomedical Applications Copyright © Rosa Estela Abreu do Nascimento, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa A Faculdade de Ciências e Tecnologia e a Universidade Nova de Lisboa têm o direito, perpétuo e sem limites geográficos, de arquivar e publicar esta dissertação através de exemplares impressos reproduzidos em papel ou de forma digital, ou por qualquer outro meio conhecido ou que venha a ser inventado, e de a divulgar através de repositórios científicos e de admitir a sua cópia e distribuição com objetivos educacionais ou de investigação, não comerciais, desde que seja dado crédito ao autor e editor. v vi Agradecimentos Agradeço à minha orientadora Dra. Luísa Neves, por me ter aceite de braços abertos e por toda a confiança depositada em mim, possibilitando-me desenvolver o projeto que lhe propus; por toda a preocupação, disponibilidade e carinho transmitidos; e pelos horizontes que me abriu para as escolhas futuras que se avizinham. -

Lectin Therapy: a Way to Explore in Order to Inhibit the Binding of COVID-19 to These Host Cells
Volume 5, Issue 5, May – 2020 International Journal of Innovative Science and Research Technology ISSN No:-2456-2165 Lectin Therapy: A Way to Explore in Order to Inhibit the Binding of COVID-19 to these Host Cells Fouad AKIF Centre Régional des Métiers de l'Éducation et de la Formation Souss Massa, B.P. 106 My. Abdellah Av. Inezegane, Morocco. Glycobiology Laboratory, Faculty of Medicine, Free University of Brussels, 808 Lennik route, 1070 Brussels, Belgium. Abstract:- When COVID-19 appeared in November highlight whatever lectins have been used for their ability 2019 in the city of Wuhan, in central China, no to block the access of certain viruses in their host cells. In epidemiologist had predicted such a pandemic. addition, we propose some lectins that can potentially be Currently, no vaccine or drug is available, the used as a possible therapy for Covid-19. treatment with azithromycin and hydroxychloroquine remains limited because it does not specifically target II. LECTINS AS ANTIVIRAL DRUGS the viral pathogen. Most enveloped viruses express glycoproteins on their surface; lectins are proteins that Any viral therapy relies on the early inhibition of viral specifically bind to glycosylated residues, many of which penetration in these target cells, and the choice of are proposed as a treatment for viral infections. Several inhibitors, the identification and characterization of the antiviral lectins are successfully used against hepatitis molecules that block the entry of the Virus are essential. C, influenza A / B, herpes, Japanese encephalitis, HIV The spread of a virus and the progression of the disease and the Ebola virus.