Oreochromis Leucostictus) Ecological Risk Screening Summary
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Impact of Climate Change at Lake Victoria
East Africa Living Lakes Network, C/O OSIENALA (Friends of Lake Victoria). Dunga Beach Kisumu Ann Nabangala Obae. Coordinator. Phone: +254-20-3588681. Email: [email protected] Background It is the Second largest fresh water lake in the World and it is surrounded by three East African States: With 6% in Kenya; Tanzania 52% and Uganda 42%. It is Located at 0:21 0 North and 3:00 0 South of the Equator Lake Victoria has a total length of 3,440 kms and 240 kms wide from East to West and is 1,134 meters above sea level with maximum depth of 82m.Its surface area is 68,870 km 2, catchment area of 180,950 km 2 Generally shallow with maximum depth of 84 meters and mean depth of 40 meters Average inflows and out flows of Lake Victoria Type of flow Flow (m3/s) Percentage (%) Inflows Rain over Lake 3,631 82 Basin Discharge 778 18 Type of flow Flow ( m3/s) Percentage (%) Out flows Evaporation from lake -3,300 76 Nile River - 1,046 24 Balance +33 Sources of Lake Victoria from Kenyan water towers Sondu Miriu river Yala river Nzoia river Mara River Kuja river Impacts and effects Changes in water budget are respectively accompanied by water level fluctuation and promote thermal structures which result in nutrient and food web dynamics. Studies have proved that there is a positive correlation between water level and fish landings (Williams, l972) The abundant fish catches are highly correlated with rainfall and lake levels. The records show that the catches reduced to between 60% and 70% during the current reduction of water level in Nyanza Gulf. -

Acte Argeo Final
GEOTHERMAL RESOURCE INDICATIONS OF THE GEOLOGIC DEVELOPMENT AND HYDROTHERMAL ACTIVITIES OF D.R.C. Getahun Demissie Addis Abeba, Ethiopia, [email protected] ABSTRACT Published sources report the occurrence of more than 135 thermal springs in D.R.C. All occur in the eastern part of the country, in association with the Western rift and the associated rifted and faulted terrains lying to its west. Limited information was available on the characteristics of the thermal features and the natural conditions under which they occur. Literature study of the regional distribution of these features and of the few relatively better known thermal spring areas, coupled with the evaluation of the gross geologic conditions yielded encouraging results. The occurrence of the anomalously large number of thermal springs is attributed to the prevalence of abnormally high temperature conditions in the upper crust induced by a particularly high standing region of anomalously hot asthenosphere. Among the 29 thermal springs the locations of which could be determined, eight higher temperature features which occur in six geologic environments were found to warrant further investigation. The thermal springs occur in all geologic terrains. Thermal fluid ascent from depth is generally influenced by faulting while its emergence at the surface is controlled by the near-surface hydrology. These factors allow the adoption of simple hydrothermal fluid circulation models which can guide exploration. Field observations and thermal water sampling for chemical analyses are recommended for acquiring the data which will allow the selection of the most promising prospects for detailed, integrated multidisciplinary exploration. An order of priorities is suggested based on economic and technical criteria. -

Departm~N • for The
Annual Report of the Game Department for the year ended 31st December, 1935 Item Type monograph Publisher Game Department, Uganda Protectorate Download date 23/09/2021 21:05:22 Link to Item http://hdl.handle.net/1834/35596 .. UqANDA PROTECTO ATE. I ANNUAL REPO T o THE • GAME DEPARTM~N • FOR THE . Year ended 31st December, 1935. I· ~nhli£heb hll ®ommanb of ll.li£ Ot.n:ellcncrr the Q301mnor. ENTEBBE: PRINTED BY THE GOVERNMENT PRINTER,. UGANDA• 1936 { .-r ~... .. , LIST OF CONTENTS. SECTION I.-ADMINISTRATION. PAGK. StaJr 3 Financial-Expenditure and Revenue 3 FOR THE DJegal Ki)ling of Game and Breaches of .Bame La"'s ... 5 Game Ordinance, 1926 5 Game Reserves. ... ... ... ... ... 5 Game Trophies, 'including Table of ,,·eight. of "hcence" ivory i SECTION Il.-ELEPHANT CO~TROL. Game Warden Game Ranger8 General Remarks 8 Return of Elephant. Destroyed ... ... •.. 8 Table d Control Ivory. based on tUok weight; and Notes 9 Clerk ... .•. J Table "(11 ,"'onnd Ivory from Uncon[,rolled are:>. ' .. 9 Tabld\'ot Faun.!! Ivory from Controlled sreas; and Notes - 9 Distritt Oont~t ... 10 1. Figures for-I General No~ r-Fatalities 18 Expenditure Elephant Speared 19 Visit to lYlasindi 'Township 19 Revenue Sex R.atio ... 19 Balanc'e 0" Curio,us Injury due to Fighting 19 Elephant Swimming 19 Nalive Tales 20 The revenue was Ri!!es ' 20 t(a) Sale of (b) Sale (c) Gam SECTION IlL-NOTES ON THE FAUNA. Receipts frolIl f\., (A) M'M~I'LS- (i) Primates 21 1934 figures; and from (il) Oarnivora 22 (iii) Ungulates 25 2; The result (8) BIRDS 30 November were quite (0) REPI'ILES 34 mately Shs. -

Living Lakes Goals 2019 - 2024 Achievements 2012 - 2018
Living Lakes Goals 2019 - 2024 Achievements 2012 - 2018 We save the lakes of the world! 1 Living Lakes Goals 2019-2024 | Achievements 2012-2018 Global Nature Fund (GNF) International Foundation for Environment and Nature Fritz-Reichle-Ring 4 78315 Radolfzell, Germany Phone : +49 (0)7732 99 95-0 Editor in charge : Udo Gattenlöhner Fax : +49 (0)7732 99 95-88 Coordination : David Marchetti, Daniel Natzschka, Bettina Schmidt E-Mail : [email protected] Text : Living Lakes members, Thomas Schaefer Visit us : www.globalnature.org Graphic Design : Didem Senturk Photographs : GNF-Archive, Living Lakes members; Jose Carlo Quintos, SCPW (Page 56) Cover photo : Udo Gattenlöhner, Lake Tota-Colombia 2 Living Lakes Goals 2019-2024 | Achievements 2012-2018 AMERICAS AFRICA Living Lakes Canada; Canada ........................................12 Lake Nokoué, Benin .................................................... 38 Columbia River Wetlands; Canada .................................13 Lake Ossa, Cameroon ..................................................39 Lake Chapala; Mexico ..................................................14 Lake Victoria; Kenya, Tanzania, Uganda ........................40 Ignacio Allende Reservoir, Mexico ................................15 Bujagali Falls; Uganda .................................................41 Lake Zapotlán, Mexico .................................................16 I. Lake Kivu; Democratic Republic of the Congo, Rwanda 42 Laguna de Fúquene; Colombia .....................................17 II. Lake Kivu; Democratic -

A BIBLIOGRAPHY of IMPORTANT TILAPIAS (PISCES: CICHLIDAE) for AQUACULTURE Oreochromisvariabilis, 0 Andersoni, 0
AMV'__ BIBLIOGRAPHIES 6 A BIBLIOGRAPHY OF IMPORTANT TILAPIAS (PISCES: CICHLIDAE) FOR AQUACULTURE Oreochromisvariabilis, 0 andersoni, 0. esculentus, 0. leucostictus, 0. rortimer, 0. spilurus niger,Sarotherodon melanotheron and Tilapia sparnmani PETER SCHOENEN INTERNATIONAL CENTER FOR LIVING AQUATIC RESOURCES MANAGEMENT A BIBLIOGRAPHY OF IMPORTANT TILAPIAS (PISCES: CICHLIDAE) FOR AQUACULTURE Oreochromls variabilis, 0. andersoni, 0. esculentus, 0. leucostictus, 0. mortimeri, 0. spilurus niger, Saro therodon melano theron and Tilapia sparrmanii Peter Schoenen International Collection "Cichlid Papers" The Referencc Service Parkstr. 15 D-5176 Inden 4 Federal Republic of Germany 1985 INTERNATIONAL CENTER FOR LIVING AQUATIC RESOURCES MANAGEMENT MANILA, PHILIPPINES A bibliography of important tilapias (Pisces: Cichlidae) for aquaculture Oreochromis variabilis, 0. andersonii, 0. esculentus, 0. leucostictus, 0. mort/tmer, 0. spilunis niger, Sarotherodon melanothero,, ard -/ilapiasparrmanii PETER SCHOENEN Published by the International Center for Living Aquatic Resources Management, MCC P.O. Box 1501, Makati, Metro Manila, Philippines with financial assistance from the International Development Research Centre of Canada through ICLARM's Selective Information Service project. 1985 Printed in Manila, Philippins This bibliography is produced directly from the author's manuscript in oider to provide tilapia workers with a useful document in the shortest time. The author should be consulted in the event of difficulty ir verifying details of particular references or in locating sources. ISSN 0115-5997 ISBN 971-1022-19-2 Schoenen, P. 1985, A bibliography of important tilapias (Pisces: Cichlidae) for aquaculture Oreochromis variabilis, 0. andersonii, 0. esculentus, 0. leucostictus, 0. mortimeri, 0. spilurut niger, Sarotherodon mela. notheron and Tilapia sparrrnanii. ICLAHM Biblio graphies 6,99 p. International Center for Living Aquatic Resources Management, Manila, Philippines. -

Implications for Management AFRICAN GREAT LAKES
AFRICAN GREAT LAKES CONFERENCE 2nd – 5th MAY 2017, ENTEBBE, UGANDA Dynamics of Fish Stocks of Commercial Importance in Lake Victoria, East Africa: Implications for Management Robert Kayanda, Anton Taabu-Munyaho, Dismas Mbabazi, Hillary Mrosso, and Chrisphine Nyamweya INTRODUCTION • Lake Victoria with a surface area of 68,800 sqkm is the world’s second largest freshwater body • It supports one of the world’s most productive inland fisheries with the estimated total fish landings from the lake for the period of 2011 to 2014 have been about 1 million tons with a beach value increasing from about US$ 550 Million in 2011 to about US$ 840 million in 2014. • It supports about 220,000 fishers (Frame Survey 2016) • The fish stocks of Lake Victoria have changed dramatically since the introduction of Nile perch Lates niloticus during the late 1950s and early 1960s Fishery Haplochromines The Original Fish Fauna Brycinus sp Protopterus Rastrineobola Mormyrus spp Barbus spp Bagrus docmac Labeo Schilbe intermedius Oreochromis variabilis Clarias gariepinus Mormyrus spp Synodontis victoriae Oreochromis leucostictus INTRODUCTION Currently, the fisheries is dominated by four major commercial important species, these are; •Nile perch •Dagaa •Nile tilapia •Haplochromis Apart from Nile tilapia only estimated through trawl and catch surveys, the other 3 are estimated through trawl, acoustics, and catch INTRODUCTION This paper summarizes current knowledge of the status of the fish stocks and reviews the need for species specific management plans for the major commercial important fish species of Lake Victoria (Nile perch, Nile tilapia, dagaa and haplochromines). Methods • Fisheries dependent – Frame surveys – Catch assessment surveys • Fisheries independent – Acoustic – Bottom trawl Biomass and relative abundance • Total biomass from the surveys 3500 remained fairly stable over time. -

Trophic Niche Segregation in the Nilotic Ichthyofauna of Lake Albert (Uganda, Africa)
Environmental Biology of Fishes (2005) 74:247–260 Ó Springer 2005 DOI 10.1007/s10641-005-3190-8 Trophic niche segregation in the Nilotic ichthyofauna of Lake Albert (Uganda, Africa) Linda M. Campbella,d, Sylvester B. Wanderab, Robert J. Thackerc,e, D. George Dixona & Robert E. Heckya aDepartment of Biology, University of Waterloo, 200 University Avenue. Waterloo, Ontario, Canada N2L 3G1 bFisheries Resources Research Institute, P.O. Box 343, Jinja, Uganda cDepartment of Physics and Astronomy, McMaster University, 1280 Main St. W, Hamilton, Ontario, Canada dCurrent address: School of Environmental Studies and Department of Biology, Queen’s University, Kingston, ON, Canada K7L 3N6 (e-mail: [email protected]) eCurrent address: Department of Physics and Astronomy, Queen’s University, Kingston, ON, Canada K7L 3N6 Received 29 April 2004 Accepted 13 February 2005 Key words: d13C, d15N, food webs, Nile perch, stable isotopes Synopsis Nile perch, Lates niloticus, and Nile tilapia, Oreochromis niloticus, were originally transplanted from Lake Albert in western Uganda to the African Great Lakes, Lake Victoria and Lake Kyoga, where they are partially implicated in reduction of the fish species diversity. Lake Albert is facing multiple environmental changes, including declining fish species diversity, hyper-eutrophication, hypoxia, and reduced fish catches. To examine the role of Nile perch and Nile tilapia in the food web in their native Lake Albert, we estimated their diets using stable nitrogen and carbon isotopes. In Lake Albert, the tilapiine congeners (closely related species), Tilapia zillii, Oreochromis leucostictus, and Sarethorodon galilaeus, and the centropomid Nile perch congener, Lates macrophthalmus, have narrower diet breath in the presence of the native O. -

Kingfisher Oil Development Surface Water
October 2017 CNOOC UGANDA LIMITED KINGFISHER OIL PROJECT, HOIMA DISTRICT, UGANDA ‐ SURFACE WATER SPECIALIST REPORT VERSION Submitted to: The Executive Director National Environment Management Authority, NEMA House, Plot 17/19/21 Jinja Road, P. O. Box 22255 Kampala, Uganda READY VOLUME 4, STUDY 2 4, STUDY VOLUME – PRINT FINAL Report Number: 1776816‐321512‐13 REPORT Distribution: 1 x electronic copy CNOOC Uganda Limited 1 x electronic copy NEMA 1 x electronic copy Eco & Partner 1 x electronic copy Golder SURFACE WATER SPECIALIST REPORT EXECUTIVE SUMMARY This report presents hydrology baseline information and an impact assessment of surface water hydrology affected by the Project. An understanding of surface water hydrological conditions prior to mine oil and gas development is essential to assess changes in water availability that could affect local users. Changes in hydrology can also affect water quality and other resources such as fish habitat, vegetation and wildlife. Hydrological data is further required to design mine oil and gas facilities (e.g. culverts, channels and storage ponds). The regional climate in the area is described as tropical with a distinct wet and dry season. Rainfall over the study area catchment varies between 700 mm and 1 400 mm/ annum. Results of Global Climate Change models indicate that Uganda is likely to experience more extreme periods of intense rainfall and drought, while the rainfall seasons become more erratic and/or infrequent. The project site is located within the Kingfisher catchment and drains westwards into the south eastern embankments of Lake Albert. Kingfisher catchment is associated with a very high western rift escarpment that drains into Lake Albert via several scattered streams and wetlands flowing westwards. -

The Charcoal Grey Market in Kenya, Uganda and South Sudan (2021)
COMMODITY REPORT BLACK GOLD The charcoal grey market in Kenya, Uganda and South Sudan SIMONE HAYSOM I MICHAEL McLAGGAN JULIUS KAKA I LUCY MODI I KEN OPALA MARCH 2021 BLACK GOLD The charcoal grey market in Kenya, Uganda and South Sudan ww Simone Haysom I Michael McLaggan Julius Kaka I Lucy Modi I Ken Opala March 2021 ACKNOWLEDGEMENTS The authors would like to thank everyone who gave their time to be interviewed for this study. They would like to extend particular thanks to Dr Catherine Nabukalu, at the University of Pennsylvania, and Bryan Adkins, at UNEP, for playing an invaluable role in correcting our misperceptions and deepening our analysis. We would also like to thank Nhial Tiitmamer, at the Sudd Institute, for providing us with additional interviews and information from South Sudan at short notice. Finally, we thank Alex Goodwin for excel- lent editing. Interviews were conducted in South Sudan, Uganda and Kenya between February 2020 and November 2020. ABOUT THE AUTHORS Simone Haysom is a senior analyst at the Global Initiative Against Transnational Organized Crime (GI-TOC), with expertise in urban development, corruption and organized crime, and over a decade of experience conducting qualitative fieldwork in challenging environments. She is currently an associate of the Oceanic Humanities for the Global South research project based at the University of the Witwatersrand in Johannesburg. Ken Opala is the GI-TOC analyst for Kenya. He previously worked at Nation Media Group as deputy investigative editor and as editor-in-chief at the Nairobi Law Monthly. He has won several journalistic awards in his career. -

———— “Mudo”: the Soga 'Little Red Riding Hood'
LILLIAN BUKAAYI TIBASIIMA ———— º “Mudo”: The Soga ‘Little Red Riding Hood’ ABSTRACT This essay analyses the social underpinnings of the oral tale of “Mudo,” which belongs to the Aarne–Thompson tale type 333, along with a group of similar tales that resemble the action and movement of “Little Red Riding Hood.” Basic to the exposition is Adolf Bastian’s assertion of the fundamental similarity of ideas between all social groups. In the “Mudo” story and its Ugandan variants, the victim is a solitary little girl and the villain a male ogre who devises ways of eating her; the ogre is mostly successful, although in some variants the girl manages to escape. Although these tales come from a great range of cultures and different geographical locations, and the counterpart of the ogre in the European tales is a wolf in disguise, they share elements of plot, characteriza- tion, and motif, and address similar concerns. Introduction USOGA IS PART OF EAS TERN UGANDA, surrounded by water. The B Rev. Fredrick Kisuule Kaliisa1 notes: To the west is river Kiira (Nile) marking the boundary between Buganda and Busoga. To the East is river Mpologoma separating Busoga from Bukedi. To the North are river Mpologoma and Lake Kyoga, forming the boundary be- tween Busoga and Lango. To the south, is Lake Victoria (Nalubaale). It might be the result of the geographical location of Busoga that ogre stories were composed to warn the people against impending harm if they went out alone and stayed in secluded places. Nnalongo Lukude emphasizes this: Historically, Busoga was surrounded by bodies of water and forests, it was very bushy and as a result harboured many wild animals, some of which were man-eaters. -
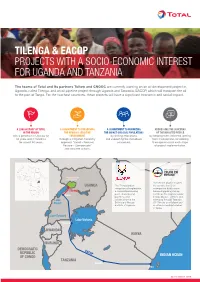
Tilenga & Eacop Projects with a Socio-Economic Interest for Uganda
TILENGA & EACOP PROJECTS WITH A SOCIO-ECONOMIC INTEREST FOR UGANDA AND TANZANIA The teams of Total and its partners Tullow and CNOOC are currently working on an oil development project in Uganda, called Tilenga, and an oil pipeline project through Uganda and Tanzania, EACOP, which will transport the oil to the port of Tanga. For the two host countries, these projects will have a significant economic and social impact. A LONG HISTORY OF TOTAL A COMMITMENT TO PRESERVING A COMMITMENT TO MINIMIZING ADDRESSING THE CONCERNS IN THE REGION THE REGION'S SENSITIVE THE IMPACT ON LOCAL POPULATIONS OF THE IMPACTED PEOPLE with a presence in Uganda for ENVIRONMENT by limiting relocations by keeping them informed, getting 60 years and in Tanzania through a mitigation hierarchy and supporting the individuals them involved and considering for almost 50 years. approach “Avoid – Reduce/ concerned. their opinions into each stage Restore – Compensate” of project implementation. and concrete actions. SOUDAN DU SUD ÉTHIOPIE Murchison Falls National Park The EACOP project involves UGANDA The Tilenga project the construction of an comprises oil exploration, underground hydrocarbon Tilenga a crude oil processing transport pipeline starting Hoima plant, underground just inside the Uganda border pipelines, and (Hoima District - 297km) and Lake infrastructure in the extending through Tanzania Albert Buliisa and Nwoya (1147km) to an oil depot and districts of Uganda. an offshore loading terminal in Tanga. Lake Edward Lake Victoria Bukoba RWANDA KENYA BURUNDI DEMOCRATIC EACOP REPUBLIC INDIAN OCEAN OF CONGO TANZANIA Singida Tanga SEPTEMBER 2019 ZAMBIE MOZAMBIQUE MALAWI SOUDAN DU SUD THIOPIE FOCUS ON THE TILENGA PROJECT Total E&P Uganda, fully aware of the project's sensitive nature, has placed particular emphasis on environmental and societal issues, with a specific commitment to leaving the site in a better state than it was before the work started and to limiting residents' relocations as much as possible. -

The Precolonial Social Formation Among the Bakenhe Fishing Community
The Precolonial Social Formation Among the Bakenhe Fishing Community .. of Lake Kyoga Il.egion of Uganda , 1800- 1894* • By C. Asowa- Okwe , Department of Political Science and Public Adminis t rat i on. Int roduction It i s eminently evident from the literqture ava ilable on the pre- colonia l history of Uganda and East Africa in general , that one area which has either been negl ected , or peripher ally treat ed i s that of the fishing industry and the fishing communities . A car eful and close examina tion of these liter a ture show a definite bia s towar ds the agricult ural and pastoral communities and their economic activities. And as if tha t is not enough , f ew that have t r i ed to grappl e with the fishing industry have l ar gely tended to be descriptive/narra tive , and ma inly, t alking a bout methods of fishing and the types of fis hes c aught. In f act the bulk of literature ~ on the fishing industry are basically , works of the physical scientists who ar e mainl y tra i ned in bi ol ogica l sciences . These studies , ther ef or e , ma i nly focus on the fish species f ound in the wa t ers of East African l akes , rivers , ponds and swamps , their f ood r equirements and dist ribut ion. They a lso concern thems elves with the question of density of fish popu- l a tion, the growth r a t e of i ndividua l species, the age at which they mature , t he specific f actor s which cause ornt~o p ic a l l ake to support many fish and another r el a tively f ew, the depletion of certa in fish species , the stocking of new species, and how t o check .the depl etion of s ome species, like·, ales.tes ( soga) , Labeo (ningu), bagrus (semutundu) .