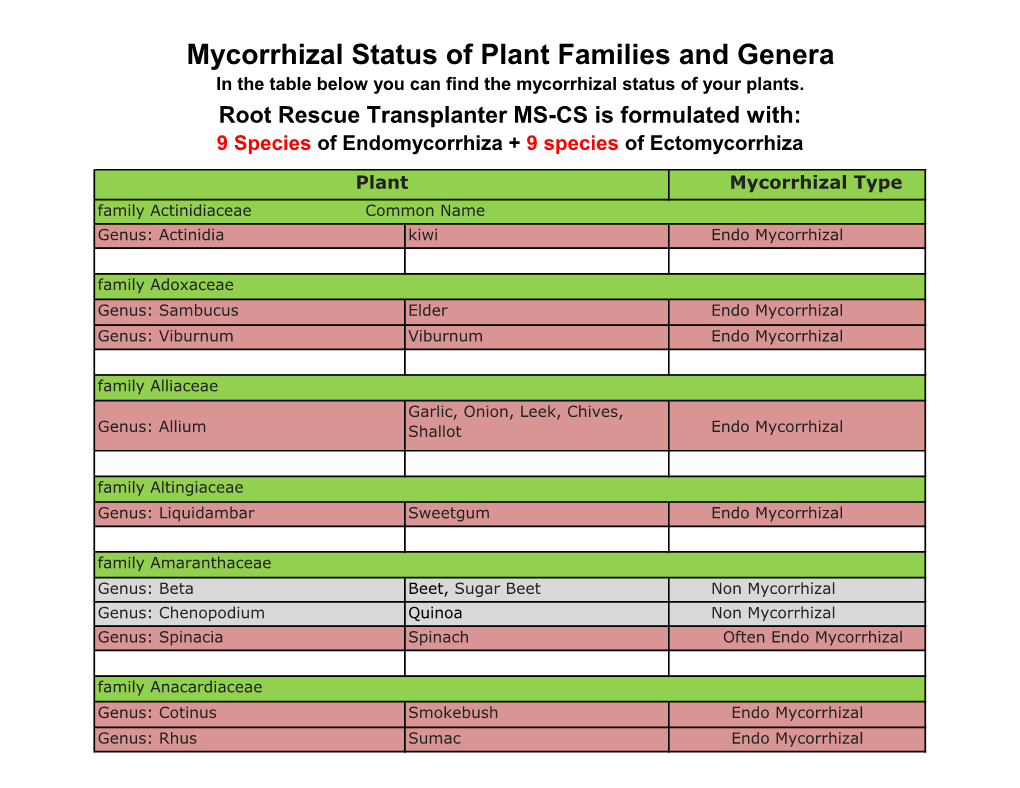
Mycorrhizal Status of Plant Families and Genera in the Table Below You Can Find the Mycorrhizal Status of Your Plants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antimicrobial and Antioxidant Activity of the Leaves, Bark and Stems of Liquidambar Styraciflua L
Int.J.Curr.Microbiol.App.Sci (2016) 5(1): 306-317 ISSN: 2319-7706 Volume 5 Number 1(2016) pp. 306-317 Journal homepage: http://www.ijcmas.com Original Research Article http://dx.doi.org/10.20546/ijcmas.2016.501.029 Antimicrobial and Antioxidant Activity of the Leaves, Bark and Stems of Liquidambar styraciflua L. (Altingiaceae) Graziele Francine Franco Mancarz1*, Ana Carolina Pareja Lobo1, Mariah Brandalise Baril1, Francisco de Assis Franco2 and Tomoe Nakashima1 1Pharmaceutical ScienceDepartment, Universidade Federal do Paraná, Curitiba, PR, Brazil 2Coodetec Desenvolvimento, Produção e Comercialização Agrícola Ltda, Cascavel, PR, Brazil *Corresponding author A B S T R A C T K e y w o r d s The genus Liquidambar L. is the best-known genus of the Altingiaceae Horan family, and species of this genus have long been used for the Liquidambar treatment of various diseases. Liquidambar styraciflua L., which is styraciflua, popularly known as sweet gum or alligator tree, is an aromatic deciduous antioxidant tree with leaves with 5-7 acute lobes and branched stems. In the present activity, study, we investigated the antimicrobial and antioxidant activity of aerial antimicrobial parts of L.styraciflua. Antimicrobial activity was evaluated using the activity, microdilution methodology. The DPPH and phosphomolybdenum methods microdilution method, were used to assess the antioxidant capacity of the samples. The extracts DPPH assay showed moderate or weak antimicrobial activity. The essential oil had the lowest MIC values and exhibited bactericidal action against Escherichia Article Info coli, Enterobacter aerogenes and Staphylococcus aureus. The ethyl acetate fraction and the butanol fraction from the bark and stem showed the best Accepted: antioxidant activity. -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

(A) Journals with the Largest Number of Papers Reporting Estimates Of
Supplementary Materials Figure S1. (a) Journals with the largest number of papers reporting estimates of genetic diversity derived from cpDNA markers; (b) Variation in the diversity (Shannon-Wiener index) of the journals publishing studies on cpDNA markers over time. Figure S2. (a) The number of publications containing estimates of genetic diversity obtained using cpDNA markers, in relation to the nationality of the corresponding author; (b) The number of publications on genetic diversity based on cpDNA markers, according to the geographic region focused on by the study. Figure S3. Classification of the angiosperm species investigated in the papers that analyzed genetic diversity using cpDNA markers: (a) Life mode; (b) Habitat specialization; (c) Geographic distribution; (d) Reproductive cycle; (e) Type of flower, and (f) Type of pollinator. Table S1. Plant species identified in the publications containing estimates of genetic diversity obtained from the use of cpDNA sequences as molecular markers. Group Family Species Algae Gigartinaceae Mazzaella laminarioides Angiospermae Typhaceae Typha laxmannii Angiospermae Typhaceae Typha orientalis Angiospermae Typhaceae Typha angustifolia Angiospermae Typhaceae Typha latifolia Angiospermae Araliaceae Eleutherococcus sessiliflowerus Angiospermae Polygonaceae Atraphaxis bracteata Angiospermae Plumbaginaceae Armeria pungens Angiospermae Aristolochiaceae Aristolochia kaempferi Angiospermae Polygonaceae Atraphaxis compacta Angiospermae Apocynaceae Lagochilus macrodontus Angiospermae Polygonaceae Atraphaxis -

H. Kiyosumiensis F
Hosta Species Update●The Hosta Library © W. George Schmid 2006 H. kiyosumiensis F. Maekawa 1935 Jornal of Japanese Botany 11:689; ic. f. 15 1935 キヨスミギボウシ = Kyosumi Giboshi H. kiyosumiensis var. petrophila F. Maekawa 1938 Divisiones et Plantae Novae Generis Hostae (2). J. Japanese Botany, 14:1:45–49. イワマ ギボウシ = Iwama Giboshi History and Nomenclature: In Japan this species is called Kyiosumi Giboshi, the “Kiyosumi (Mountain) Hosta.” The species epithet stems from the Latinized name of Kiyosumiyama (清澄山), a small mountain (380 m/about 1250 feet AMSL with a great view of Tokyo Bay) located on Boso Peninsula (Bōsō-hantō; 房総半島), which is located in south Chiba Prefecture (Chiba-ken; 千葉県) forming the eastern edge of Tokyo Bay. Maekawa established H. kiyosumiensis in 1935 and published further on the species in 1938, when he also described a new variety of the species as H. (kiyosumiensis var.) petrophila. This varietal epithet is derived from the Latin “liking (or) growing on rocks.” The Japanese name Iwama Giboshi (イワマ ギボウシ) has the same meaning. The latter is differentiated by minor morphological details caused by its different, rocky habitat in Yamashiro province (山城国; Yamashiro-no kuni), H. kiyosumiensis Maekawa 1935 (in situ) Otogawa (男川) River; Okazaki-shi (岡崎市; formerly Nukata-cho) Aichi Prefecture (愛知県; Aichi-ken) 1 an old province of Japan (today the southern part of Kyoto Prefecture). Maekawa (1938) gave a much abbreviated Latin description and the variety is here considered synonymous with the species. In fact, Maekawa (1969) no longer supported the varietal rank. N. Fujita (1976) confirmed H. kiyosumiensis as a species and added two morphs previously described by Maekawa (1940), i.e., H. -

Tree of the Year: Liquidambar Eric Hsu and Susyn Andrews
Tree of the Year: Liquidambar Eric Hsu and Susyn Andrews With contributions from Anne Boscawen (UK), John Bulmer (UK), Koen Camelbeke (Belgium), John Gammon (UK), Hugh Glen (South Africa), Philippe de Spoelberch (Belgium), Dick van Hoey Smith (The Netherlands), Robert Vernon (UK) and Ulrich Würth (Germany). Affinities, generic distribution and fossil record Liquidambar L. has close taxonomic affinities with Altingia Noronha since these two genera share gum ducts associated with vascular bundles, terminal buds enclosed within numerous bud scales, spirally arranged stipulate leaves, poly- porate (with several pore-like apertures) pollen grains, condensed bisexual inflorescences, perfect or imperfect flowers, and winged seeds. Not surpris- ingly, Liquidambar, Altingia and Semiliquidambar H.T. Chang have now been placed in the Altingiaceae, as originally treated (Blume 1828, Wilson 1905, Chang 1964, Melikan 1973, Li et al. 1988, Zhou & Jiang 1990, Wang 1992, Qui et al. 1998, APG 1998, Judd et al. 1999, Shi et al. 2001 and V. Savolainen pers. comm.). These three genera were placed in the subfamily Altingioideae in Hamamelidaceae (Reinsch 1890, Chang 1979, Cronquist 1981, Bogle 1986, Endress 1989) or the Liquidambaroideae (Harms 1930). Shi et al. (2001) noted that Altingia species are evergreen with entire, unlobed leaves; Liquidambar is deciduous with 3-5 or 7-lobed leaves; while Semiliquidambar is evergreen or deciduous, with trilobed, simple or one-lobed leaves. Cytological studies have indicated that the chromosome number of Liquidambar is 2n = 30, 32 (Anderson & Sax 1935, Pizzolongo 1958, Santamour 1972, Goldblatt & Endress 1977). Ferguson (1989) stated that this chromosome number distinguished Liquidambar from the rest of the Hamamelidaceae with their chromosome numbers of 2n = 16, 24, 36, 48, 64 and 72. -

Hamamelidaceae (& Altingiaceae*)
Hamamelidaceae (& Altingiaceae*) Altingia Noronha* Loropetalum R.Br. ex Rchb. Corylopsis Siebold & Zucc. Parrotia C.A.Mey. Disanthus Maxim. Parrotiopsis (Nied.) C.K.Schneid. Distylium Siebold & Zucc. Rhodoleia Champ. ex Hook. Exbucklandia R.W.Br. Sinowilsonia Hemsl. Fortunearia Rehder & E.H.Wilson ×Sycoparrotia Endress & Anliker Fothergilla L. Sycopsis Oliv. Hamamelis L. Trichocladus Pers. 1 Liquidambar L.* Uocodendron VEGETATIVE KEY TO SPECIES CULTIVATED IN WESTERN EUROPE Jan De Langhe (29 July 2012 - 9 May 2014) Vegetative key. This key is based on vegetative characteristics, and therefore also usable beyond the flowering/fruiting period. Taxa treated in this key: see page 7. Taxa referred to synonymy in this key: see page 7. Questionable/freguently misapplied names: see page 7. To improve accuracy: - Use a hand lens to judge pubescence in general. - Start counting veins at base of the lamina with first clearly ascending secondary vein, do not include veins ending in the apex. - Look at the entire plant. Young specimens and strong shoots give an atypical view. - Beware of hybridisation, especially with plants raised from seed gathered in collections. Background information: - JDL herbarium specimens - living specimens, in various arboreta, botanic gardens and collections - selected literature: Andrews, S. & Hsu, E. - (2004) - Liquidambar as Tree of the Year in IDS yearbook, p.11-45. Bean, W.J. - (1980) - Corylopsis in Trees and Shrubs hardy in the British Isles VOL.1, p.717-721. Bean, W.J. - (1981) - Disanthus in Trees and Shrubs hardy in the British Isles VOL.2, p.62. Bean, W.J. - (1981) - Distylium in Trees and Shrubs hardy in the British Isles VOL.2, p.65-66. -

Description and Identification of Ostryopsis Davidiana Ectomycorrhizae in Inner Mongolia Mountain Forest of China
Österr. Z. Pilzk. 26 (2017) – Austrian J. Mycol. 26 (2017) 17 Description and identification of Ostryopsis davidiana ectomycorrhizae in Inner Mongolia mountain forest of China QING-ZHI YAO1 WEI YAN2 HUI-YING ZHAO1 JIE WEI2 1 Life Science College 2 Forestry College Inner Mongolia Agriculture University Huhhot, 010018, P. R. China Email: [email protected] Accepted 27. March 2017. © Austrian Mycological Society, published online 23. August 2017 YAO, Q.-Z., YAN, W., ZHAO, H.-Y., WEI, J., 2017: Description and identification of Ostryopsis davidi- ana ectomycorrhizae in Inner Mongolia mountain forest of China. – Austrian J. Mycol. 26: 17–25. Key words: ECM, Mountain forest, Ostryopsis davidiana, morpho-anatomical features. Abstract: The ectomycorrhizal (ECM) fungal composition and anatomical structures of root samples of the shrub Ostryopsis davidiana were examined. The root samples were collected from two plots in the Daqing Mountain and Han Mountain around Hohhot, Inner Mongolia of China. Basing on mor- pho-anatomical features of the samples, we have got totally 12 ECM morphotypes. Twelve fungal taxa were identified via sequencing of the internal transcribed spacer region of their nuclear rDNA. Nine species are Basidiomycotina, incl. Thelephoraceae (Tomentella), Cortinariaceae (Inocybe and Cortinarius), Tremellaceae (Sebacina), Russulaceae (Lactarius), and Tricholomataceae (Tricholoma), three Ascomycotina, incl. Elaphomycetaceae (Cenococcum), Tuberaceae (Tuber), and Pyronema- taceae (Wilcoxina). Cenococcum geophilum was the dominant species in O. davidiana. The three To- mentella and the two Inocybe ECMF of O. davidiana are very common in Inner Mongolia. Zusammenfassung: Die Pilzdiversität der Ektomykorrhiza (ECM) und deren anatomische Strukturen von Wurzelproben des Strauches Ostryopsis davidiana wurden untersucht. Die Wurzelproben wurden aus zwei Untersuchungsflächen im Daqing Berg und Han Berg nahe Hohhot, Innere Mongolei, China, gesammelt. -

Diploid Hybrid Origin of Ostryopsis Intermedia (Betulaceae) in the Qinghai-Tibet Plateau Triggered by Quaternary Climate Change
Molecular Ecology (2014) 23, 3013–3027 doi: 10.1111/mec.12783 Diploid hybrid origin of Ostryopsis intermedia (Betulaceae) in the Qinghai-Tibet Plateau triggered by Quaternary climate change BINGBING LIU,*† RICHARD J. ABBOTT,‡ ZHIQIANG LU,† BIN TIAN† and JIANQUAN LIU* *MOE Key Laboratory of Bio-Resources and Eco-Environment, College of Life Science, Sichuan University, Chengdu 610065, China, †State Key Laboratory of Grassland Agro-Ecosystem, College of Life Science, Lanzhou University, Lanzhou 730000, China, ‡School of Biology, University of St Andrews, Mitchell Building, St Andrews, Fife, KY16 9TH, UK Abstract Despite the well-known effects that Quaternary climate oscillations had on shaping intraspecific diversity, their role in driving homoploid hybrid speciation is less clear. Here, we examine their importance in the putative homoploid hybrid origin and evolu- tion of Ostryopsis intermedia, a diploid species occurring in the Qinghai-Tibet Plateau (QTP), a biodiversity hotspot. We investigated interspecific relationships between this species and its only other congeners, O. davidiana and O. nobilis, based on four sets of nuclear and chloroplast population genetic data and tested alternative speciation hypotheses. All nuclear data distinguished the three species clearly and supported a close relationship between O. intermedia and the disjunctly distributed O. davidiana. Chloroplast DNA sequence variation identified two tentative lineages, which distin- guished O. intermedia from O. davidiana; however, both were present in O. nobilis. Admixture analyses of genetic polymorphisms at 20 SSR loci and sequence variation at 11 nuclear loci and approximate Bayesian computation (ABC) tests supported the hypothesis that O. intermedia originated by homoploid hybrid speciation from O. davidiana and O. -

Reducing Deer Damage in Landscapes Part 2
Reducing Deer Damage in the Landscape D Coetzee Public Domain bestfriendthemom Dusty Hancock CC BY-NC-ND 2.0 Chatham Master Gardener Volunteer Matt Jones Horticulture Agent NC Cooperative Extension - Chatham County Center Plant Selection Deer Candy • Aucuba • Hosta • Arborvitae • Indian Hawthorn • Azalea • Ivy • Blueberry • Chionanthus, • Clematis Malus, Prunus, • Daylily Pyrus • Euonymus • Redbuds • Fatsia • Roses Muhenbergia capillaris Pink Muhly Grass (Poaceae) Andrea Laine Jim Robbins CC BY NC 4.0 CC BY-NC-ND 4.0 Muhlenbergia capillaris Pink Muhly Grass (Poaceae) Full Sun Moist to very dry 1-3’ x 1-3’ Fall Susan Strine Fall-Winter CC BY 2.0 Schizachyrium scoparium Little Blue Stem (Poaceae) Jim Robbins Joshua Mayer Jim Robbins Montreais CC BY-SA 2.0 DE CC BY-NC-ND 4.0 CC BY-NC-ND 4.0 CC BY-SA 3.0 Schizachyrium scoparium Little Blue Stem (Poaceae) Full Sun Moist to dry Good drainage 1-4’ x 18”-2’ Summer-Fall Susan Strine Summer-Fall Jim RobbinsCC BY 2.0 CC BY-NC-ND 4.0 Chasmanthium latifolium River Oats (Poaceae) Klasse im Garten Anne McCormack CC BY 2.0 CC BY-NC 2.0 Chasmanthium latifolium River Oats (Poaceae) Part shade to dappled sun Moist Occasionally wet 2-5’ x 1-2’ Summer-Fall Anne McCormack Summer-Fall CC BY-NC 2.0 Myrica cerifera (Myricaceae) Common Wax Myrtle Forest and Kim Starr CC BY 2.0 Forest and Kim Starr CC BY 2.0 Myrica cerifera (Myricaceae) Common Wax Myrtle Jim Robbins CC BY-NC-ND 4.0 Jim Robbins Jim Robbins CC BY-NC-ND 4.0 CC BY-NC-ND 4.0 Great for urban soils, full sun to part shade. -

Integrating Palaeontological and Molecular Data Uncovers Multiple
Integrating palaeontological and molecular data uncovers multiple ancient and recent dispersals in the pantropical Hamamelidaceae Xiaoguo Xiang, Kunli Xiang, Rosa del C. Ortiz, Florian Jabbour, Wei Wang To cite this version: Xiaoguo Xiang, Kunli Xiang, Rosa del C. Ortiz, Florian Jabbour, Wei Wang. Integrating palaeontolog- ical and molecular data uncovers multiple ancient and recent dispersals in the pantropical Hamamel- idaceae. Journal of Biogeography, Wiley, 2019, 46 (11), pp.2622-2631. 10.1111/jbi.13690. hal- 02612865 HAL Id: hal-02612865 https://hal.archives-ouvertes.fr/hal-02612865 Submitted on 19 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Integrating palaeontological and molecular data uncovers multiple ancient and recent dispersals in the pantropical Hamamelidaceae Xiaoguo Xiang1,2, Kunli Xiang1,3, Rosa Del C. Ortiz4, Florian Jabbour5, Wei Wang1,3 1State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing, China 2Jiangxi Province Key Laboratory of Watershed Ecosystem -

Global Survey of Ex Situ Betulaceae Collections Global Survey of Ex Situ Betulaceae Collections
Global Survey of Ex situ Betulaceae Collections Global Survey of Ex situ Betulaceae Collections By Emily Beech, Kirsty Shaw and Meirion Jones June 2015 Recommended citation: Beech, E., Shaw, K., & Jones, M. 2015. Global Survey of Ex situ Betulaceae Collections. BGCI. Acknowledgements BGCI gratefully acknowledges the many botanic gardens around the world that have contributed data to this survey (a full list of contributing gardens is provided in Annex 2). BGCI would also like to acknowledge the assistance of the following organisations in the promotion of the survey and the collection of data, including the Royal Botanic Gardens Edinburgh, Yorkshire Arboretum, University of Liverpool Ness Botanic Gardens, and Stone Lane Gardens & Arboretum (U.K.), and the Morton Arboretum (U.S.A). We would also like to thank contributors to The Red List of Betulaceae, which was a precursor to this ex situ survey. BOTANIC GARDENS CONSERVATION INTERNATIONAL (BGCI) BGCI is a membership organization linking botanic gardens is over 100 countries in a shared commitment to biodiversity conservation, sustainable use and environmental education. BGCI aims to mobilize botanic gardens and work with partners to secure plant diversity for the well-being of people and the planet. BGCI provides the Secretariat for the IUCN/SSC Global Tree Specialist Group. www.bgci.org FAUNA & FLORA INTERNATIONAL (FFI) FFI, founded in 1903 and the world’s oldest international conservation organization, acts to conserve threatened species and ecosystems worldwide, choosing solutions that are sustainable, based on sound science and take account of human needs. www.fauna-flora.org GLOBAL TREES CAMPAIGN (GTC) GTC is undertaken through a partnership between BGCI and FFI, working with a wide range of other organisations around the world, to save the world’s most threated trees and the habitats which they grow through the provision of information, delivery of conservation action and support for sustainable use. -

Phylogeographical Structure of Liquidambar Formosana Hance Revealed by Chloroplast Phylogeography and Species Distribution Models
Article Phylogeographical Structure of Liquidambar formosana Hance Revealed by Chloroplast Phylogeography and Species Distribution Models 1,2, 1, 1 2 1 1, Rongxi Sun y , Furong Lin y, Ping Huang , Xuemin Ye , Jiuxin Lai and Yongqi Zheng * 1 State Key Laboratory of Tree Genetics and Breeding, Key Laboratory of Silviculture of the State Forestry Administration, Research Institute of Forestry, Chinese Academy of Forestry, Beijing 100091, China; [email protected] (R.S.); [email protected] (F.L.); [email protected] (P.H.); [email protected] (J.L.) 2 Jiangxi Provincial Key Laboratory of Silviculture, College of Forestry, Jiangxi Agricultural University, Nanchang 330045, China; [email protected] * Correspondence: [email protected]; Tel.: +86-10-6288-8565 These authors contributed equally to this work. y Received: 2 September 2019; Accepted: 29 September 2019; Published: 1 October 2019 Abstract: To understand the origin and evolutionary history, and the geographical and historical causes for the formation of the current distribution pattern of Lquidambar formosana Hance, we investigated the phylogeography by using chloroplasts DNA (cpDNA) non-coding sequences and species distribution models (SDM). Four cpDNA intergenic spacer regions were amplified and sequenced for 251 individuals from 25 populations covering most of its geographical range in China. A total of 20 haplotypes were recovered. The species had a high level of chloroplast genetic variation (Ht = 0.909 0.0192) and a significant phylogeographical structure (genetic differentiation takes into ± account distances among haplotypes (Nst) = 0.730 > population differentiation that does not consider distances among haplotypes (Gst) = 0.645; p < 0.05), whereas the genetic variation within populations (Hs = 0.323 0.0553) was low.