Informed Consent Form Approved on 10/12/2017
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Key Features of Candesartan Cilexetil and a Comparison with Other Angiotensin II Receptor Antagonists
Journal of Human Hypertension (1999) 13, (Suppl 1), S3–S10 1999 Stockton Press. All rights reserved 0950-9240/99 $12.00 Key features of candesartan cilexetil and a comparison with other angiotensin II receptor antagonists PS Sever Imperial College of Science, Technology & Medicine at St Mary’s Hospital, London, UK Current research on angiotensin II AT1-receptor antag- in patients with essential hypertension. Candesartan onists (AIIRAs) and selected studies presented at the cilexetil has a rapid onset of action (approximately 80% recent symposium held in Amsterdam, The Netherlands, of total blood pressure reduction within the first 2 on 6 June 1998, titled ‘Angiotensin II Receptor Antagon- weeks) and dose-dependent effects on blood pressure, ists are NOT all the Same’ are reviewed. AIIRAs offer a is comparable in efficacy to a number of classes of anti- number of potential advantages over alternative antihy- hypertensives, and is effective in combination therapy pertensive agents acting via the renin-angiotensin-aldo- (eg, with hydrochlorothiazide and amlodipine). This sterone system. They combine blood pressure-lowering favourable profile may be due in part to the highly selec- effects at least equivalent to those of angiotensin-con- tive, tight binding to and slow dissociation of candesar- verting enzyme (ACE) inhibitors, coupled with placebo- tan from the AT1 receptor. Preliminary studies suggest like tolerability. Candesartan cilexetil is a novel AIIRA that candesartan cilexetil also protects end organs that has demonstrated clinical -
Download Leaflet View the Patient Leaflet in PDF Format
Package leaflet: Information for the user Candesartan cilexetil 2 mg tablets Candesartan cilexetil 4 mg tablets Candesartan cilexetil 8 mg tablets Candesartan cilexetil 16 mg tablets Candesartan cilexetil 32 mg tablets candesartan cilexetil Read all of this leaflet carefully If you are going to have an operation, before you start taking this tell your doctor or dentist that you are medicine because it contains taking Candesartan cilexetil. This is important information for you. because Candesartan cilexetil, when - Keep this leaflet. You may need combined with some anaesthetics, to read it again. may cause an excessive drop in - If you have any further questions, blood pressure. ask your doctor or pharmacist. - This medicine has been Children and adolescents prescribed for you only. Do not Candesartan Cilexetil has been pass it on to others. It may harm studied in children. For more them, even if their signs of illness information, talk to your doctor. are the same as yours. Candesartan Cilexetil must not be - If you get any side effects, talk to given to children under 1 year of your doctor or pharmacist. This age due to the potential risk to the includes any possible side effects developing kidneys. not listed in this leaflet. See Other medicines and Candesartan section 4. cilexetil What is in this leaflet Tell your doctor or pharmacist if you 1. What Candesartan cilexetil is and are taking, have recently taken or what it is used for might take any other medicines. 2. What you need to know before you take Candesartan cilexetil Candesartan cilexetil can affect the 3. -

Blocking the Tissue Renin-Angiotensin System: the Future Cornerstone of Therapy
Journal of Human Hypertension (2000) 14, Suppl 2, S23–S31 2000 Macmillan Publishers Ltd All rights reserved 0950-9240/00 $15.00 www.nature.com/jhh Blocking the tissue renin-angiotensin system: the future cornerstone of therapy T Unger1, M Azizi2 and GG Belz3 1Institute of Pharmacology, Christian Albrechts University, Hospitalstrae 4, 24105 Kiel, Germany; 2Centre D’Investigations Cliniques, Hoˆ pital Broussais, 96 Rue Didot – 75674, Paris Cedex 14, France; 3Zentrum fuer Kardiovaskulaere Pharmakologie, ZeKaPha GmbH, Mathildenstrae 8, D-55116, Mainz-Wiesbaden, Germany The development of angiotensin-converting enzyme antagonist, is characterised by its tight binding to and inhibitors and selective angiotensin type 1 (AT1)- slow dissociation from the AT1 receptor, and high antag- receptor antagonists has provided new insights into onistic potency, resulting in long-lasting antagonistic understanding the mechanism of the renin-angiotensin effects. It is anticipated that these pharmacological system (RAS) in the pathophysiology of cardiovascular characteristics may bring additional benefits to patients, disease. There is good evidence from meta-analyses not only for the management of essential hypertension that shows that inhibition of the RAS achieves organ but also for the management of end-organ damage. protection features that go beyond blood pressure con- Journal of Human Hypertension (2000) 14, Suppl 2, S23– trol. Candesartan cilexetil, a new angiotensin II receptor S31 Keywords: renin-angiotensin system; angiotensin-converting enzyme inhibitor; angiotensin type 1 receptor; angiotensin receptor antagonist; candesartan cilexetil Introduction Blocking the RAS in essential Hypertension is a major risk factor for myocardial hypertension infarction, stroke, and renal and peripheral vascular Angiotensin II, the key effector peptide of the RAS, disease. -
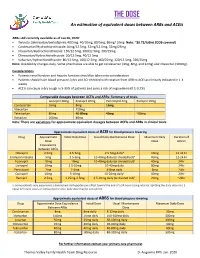
THE DOSE an Estimation of Equivalent Doses Between Arbs and Aceis
THE DOSE An estimation of equivalent doses between ARBs and ACEIs ARBs still currently available as of Jan 26, 2020: Twynsta (telmisartan/amlodipine): 40/5mg. 40/10mg, 80/5mg, 80mg/ 10mg Note: ~$0.73/tablet (ODB covered) Candesartan/Hydrochlorothiazide:16mg/12.5mg, 32mg/12.5mg, 32mg/25mg Irbesartan/Hydrochlorothiazide: 150/12.5mg, 300/12.5mg, 300/25mg Olmesartan/Hydrochlorothiaizde: 20/12.5mg, 40/12.5mg Valsartan/Hydrochlorothiazide: 80/12.5mg, 160/12.5mg, 160/25mg, 320/12.5mg, 320/25mg Note: Availability changes daily. Some pharmacies are able to get candesartan (4mg, 8mg, and 32mg) and irbesartan (300mg). Considerations Patients renal function and hepatic function should be taken into consideration Patients should have blood pressure, lytes and SCr checked with rotation from ARB to ACEI as clinically indicated in 1-4 weeks ACEIs can cause a dry cough in 5-35% of patients and carry a risk of angioedema (0.1-0.2%) Comparable dosages between ACEIs and ARBs- Summary of trials Lisinopril 20mg Enalapril 20mg Perindopril 4mg Ramipril 10mg Candesartan 16mg 8mg 16mg Irbesartan 150mg Telmisartan 80mg 40-80mg 40mg ~80mg Valsartan 160mg 80mg Note: There are variations for approximate equivalent dosages between ACEIs and ARBs in clinical trials. Approximate equivalent doses of ACEI for blood pressure lowering Drug Approximate Initial Daily Dose Usual Daily Maintenance Dose Maximum Daily Duration of Dose Dose Action Equivalence Between ACEIs Cilazapril 2.5mg 2.5-5mg 2.5-5mg dailya 10mg 12-24 hr Enalapril maleate 5mg 2.5-5mg 10-40mg daily (or divided bid)a 40mg 12-24 hr Fosinopril 10mg 10mg 10-40mg daily (or divided bid)a 40mg 24hr Lisinopril 10mg 2.5-10mg 10-40mg daily 80mg 24hr Perindopril 2mg 2-4mg 4-8mg daily 8mg 24hr Quinapril 10mg 5-10mg 10-20mg dailya 40mg 24hr Ramipril 2.5mg 1.25mg-2.5mg 2.5-10mg daily (or divided bid)a 20mg ~24hr a: Some patients may experience a diminished antihypertensive effect toward the end of a 24-hour dosing interval. -

Comparison of the Efficacy of Candesartan and Losartan: a Meta-Analysis of Trials in the Treatment of Hypertension
Journal of Human Hypertension (2010) 24, 525–531 & 2010 Macmillan Publishers Limited All rights reserved 0950-9240/10 www.nature.com/jhh ORIGINAL ARTICLE Comparison of the efficacy of candesartan and losartan: a meta-analysis of trials in the treatment of hypertension PA Meredith, LS Murray and GT McInnes Department of Medicine & Therapeutics, Division of Cardiovascular & Medical Sciences, Gardiner Institute, Western Infirmary, Glasgow, Scotland, UK Informed by the findings from prospective observational included in the analysis using a random effect model. studies and randomized outcome trials, guidelines for Mean changes in SBP and DBP were compared for each the management of hypertension acknowledge that drug alone and after stratification for dose and for the benefit of treatment can be attributed largely to combination with hydrochlorothiazide (HCTZ). On the blood pressure (BP) reduction. Therefore, quantification basis of all the data, the weighted mean difference of differential BP lowering of different agents within favoured candesartan—3.22 mm Hg (95% confidence classes of anti-hypertensives is of practical importance. interval (CI) 2.16, 4.29) for SBP and 2.21 mm Hg (95% CI The objective of this analysis was to compare the 1.34, 3.07) for DBP. These findings were consistent efficacy of candesartan and losartan with respect to when analyses according to dose and combination with reduction in systolic and diastolic BP (SBP and DBP). HCTZ were carried out. Thus, it can be concluded that at A systematic literature search of databases from 1980 to currently recommended doses, candesartan is more 1 October 2008 identified 13 studies in which candesartan effective than losartan in lowering BP. -

Shortage of Valsartan Formulations
Shortage of Valsartan Formulations Updated 24th September 2019 Description of products affected Valsartan is an angiotensin II receptor antagonist (AIIRA) licensed for the treatment of patients with: • Hypertension. • Symptomatic heart failure. • Post- myocardial infarction (MI) with left ventricular failure or left ventricular systolic dysfunction (LVSD). Licensed doses range from 40mg to 320mg per day given once or twice daily depending on indication. It is one of several AIIRAs on the UK market which are all licensed for the treatment of hypertension but differ in their other licensed indications. Background • There have been intermittent supply problems with all strengths of valsartan formulations (including both capsules and tablets). • Our Medicines Optimisation Team has managed to ascertain the following availability from manufacturers as of 20th September 2019: o Crescent – Valsartan 80mg capsules and valsartan 160mg capsules are available. o DE Pharmaceuticals – Valsartan 40mg tablets and valsartan 320mg tablets are available. o Mylan – Valsartan 160mg capsules and valsartan 320mg tablets are available. • There is currently only the broad anticipated resolution date of October to November 2019. • Suppliers of alternative AIIRAs have been contacted to determine if they have sufficient stock availability and currently both Losartan and Candesartan are available from the 3 major wholesalers: AAH, Alliance HC and Phoenix. Alternative agents and suggested management options • The patient should be encouraged to try several pharmacies in order to fulfil the prescription as different pharmacies use a range of wholesalers and distributors. The patient may wish to ring pharmacies in advance of attending to ascertain availability. Page 1 of 3 • The decision about what to do will need to be individualised to each patient. -

Comparison of the AT1-Receptor Blocker, Candesartan Cilexetil, And
Journal of Human Hypertension (2000) 14, 263–269 2000 Macmillan Publishers Ltd All rights reserved 0950-9240/00 $15.00 www.nature.com/jhh ORIGINAL ARTICLE Comparison of the AT1-receptor blocker, candesartan cilexetil, and the ACE inhibitor, lisinopril, in fixed combination with low dose hydrochlorothiazide in hypertensive patients GT McInnes1, KPJ O’Kane1, H Istad2, S Keina¨nen-Kiukaanniemi3 and HFCM Van Mierlo4 1University Department of Medicine and Therapeutics Western Infirmary, Glasgow G11 6NT, UK; 2Theresegt, Legesenter, Theresegt. 35B, 0354 Oslo, Norway; 3Department of Public Health Science and General Practice, Aapistie 1, 90220 Oula, Finland; 4Rembrantsplein 7, 2377 BM Oude Wetering, The Netherlands Aim: To compare candesartan cilexetil and lisinopril in ables (sitting systolic blood pressure, standing blood fixed combination with hydrochlorothiazide with respect pressure, sitting/erect heart rate, and proportion of to antihypertensive efficacy and tolerability. responders and controlled patients). Both drugs were Methods: This was a double-blind (double-dummy), ran- well tolerated but the proportion of patients with at least domised, parallel group comparison in patients with a one adverse event was significantly greater in the lisino- The proportion of .(0.020 ؍ mean sitting diastolic blood pressure 95–115 mm Hg on pril group (80% vs 69%, P prior antihypertensive monotherapy. Treatments were patients spontaneously reporting cough (23.1% vs 4.6%) candesartan cilexetil/hydrochlorothiazide 8/12.5 mg and discontinuing therapy due to adverse events (12.0% -and lisinopril/hydrochlorothiazide vs 5.9%) was also higher in the lisinopril group com (237 ؍ once daily (n .for 26 weeks. The pri- pared with the candesartan cilexetil group (116 ؍ mg once daily (n 10/12.5 mary efficacy variable was change in trough sitting dia- Conclusions: The fixed combinations of candesartan stolic blood pressure. -

AT1-Receptor Blockers: Differences That Matter
Journal of Human Hypertension (2002) 16, S9–S16 2002 Nature Publishing Group All rights reserved 0950-9240/02 $25.00 www.nature.com/jhh AT1-receptor blockers: differences that matter AH Gradman Division of Cardiovascular Diseases, The Western Pennsylvania Hospital, Pittsburgh, Pennsylvania, USA The available angiotensin II type 1 (AT1)-receptor block- effect, and this finding is supported by the results of ers differ markedly in their pharmacological properties comparative clinical trials. Moreover, the prolonged and clinical efficacy. Losartan shifts the dose-response binding of candesartan to the receptor is reflected in a curve for angiotensin II to the right without affecting the longer duration of action, compared with losartan; the maximal response; this antagonism can be overcome by antihypertensive effect of candesartan persists for 48 h increasing concentrations of angiotensin II and thus los- after dosing, compared with approximately 24 h with artan acts as a surmountable antagonist. By contrast, losartan. Candesartan thus offers extended therapeutic other agents suppress the maximal response to angio- coverage, an important consideration since a majority tensin II to varying extents; this can not be overcome by of patients miss occasional doses of antihypertensive increasing angiotensin concentrations and hence these medication. There is currently no evidence that differ- agents are insurmountable antagonists. Receptor bind- ences in receptor binding between AT1-receptor block- ing studies have shown that candesartan has the high- ers translate into differences in tolerability. In summary, est affinity for the AT1-receptor, followed by irbesartan, therefore, pharmacological differences between AT1- valsartan and losartan, and that candesartan dis- receptor blockers are reflected in clinically important sociates from the receptor more slowly than other differences in maximal antihypertensive effect, antagonists. -

Candesartan Cilexetil – Atacand , Astra Merck Development And
Candesartan cilexetil – AtacandÒ, Astra Merck Development and Pharmacology:1-3 The renin-angiotensin-aldosterone system (RAAS) plays a critical role in cardiovascular and renal function and therefore in the regulation of blood pressure. Renin, a proteinase enzyme, is secreted by the kidney in response to a reduction in renal blood flow, blood pressure or sodium concentration. Renin converts angiotensinogen, which is secreted by the liver, to the decapeptide angiotensin I (AI). AI is cleaved by angiotensin converting enzyme (ACE) to the octapeptide angiotensin II (AII). AII produces potent vasoconstriction via interaction with vascular angiotensin receptors (AT1 receptors). AII also promotes aldosterone secretion and therefore sodium retention by stimulation of AT1 receptors present on the adrenal cortex. These actions result in elevated blood pressure secondary to the vasoconstriction and enhanced cardiac output secondary to sodium retention. In addition to its normal regulatory role, the RAAS also contributes to pathological conditions such as renovascular hypertension, essential hypertension and congestive heart failure. Over the past several decades research efforts have been directed toward developing drugs capable of suppressing of RAAS by inhibiting renin release, blocking the formation of AII via inhibition of ACE, or antagonism of AII at its physiologic receptors. These efforts led first to the introduction of clinically effective ACE inhibitors (ACEIs), beginning with captopril in 1981 and followed by enalapril (Vasotec), lisinopril (Prinivil, Zestril), benazepril (Lotensin), fosinopril (Monopril), quinapril (Accupril), ramipril (Altace), moexipril (Univasc) and trandolapril (Mavik). ACEIs have been successfully employed in the management of various forms of hypertension as well as congestive heart failure. However, ACE has other physiologic actions not related to the regulation of RAAS, including the degradation of bradykinin and other peptides including substance P. -

Comparison of Valsartan with Candesartan on Their Possible Protection from Atherosclerosis
Journal of Clinical and Basic Cardiology An Independent International Scientific Journal Journal of Clinical and Basic Cardiology 2001; 4 (4), 297-299 Comparison of Valsartan With Candesartan on Their Possible Protection From Atherosclerosis Mueck AO, Heuberger H, Seeger H, Wallwiener D Homepage: www.kup.at/jcbc Online Data Base Search for Authors and Keywords Indexed in Chemical Abstracts EMBASE/Excerpta Medica Krause & Pachernegg GmbH · VERLAG für MEDIZIN und WIRTSCHAFT · A-3003 Gablitz/Austria ORIGINAL PAPERS, BASIC CARDIOLOGY Valsartan, Candesartan and Protection from Atherosclerosis J Clin Basic Cardiol 2001; 4: 297 Comparison of Valsartan With Candesartan on Their Possible Protection From Atherosclerosis A. O. Mueck, H. Seeger, W. Heuberger, D. Wallwiener It is well appreciated that AT1-antagonists do diminish long-term effects of angiotensin on the blood pressure which are regarded as detrimental. In the present in vitro experiments we compared the efficacies of valsartan and candesartan in prevent- ing negative outcomes of angiotensin effect on markers of endothelial function and on proliferation of smooth muscle cells. Angiotensin II (10 µM) induced a decrease in the concentration of endothelial-derived nitric oxide synthase and increases the concentration of the vasoconstrictor endothelin, the procoagulatory substance plasminogen-activator-inhibitor-1 (PAI-1) and of the precursor of the matrix-metalloproteinase 1 (MMP-1) in endothelial cell cultures from human coronary arteries. These changes were completely prevented by the addition of 10 µM of valsartan or candesartan and partially by the addition of lower concentrations of the sartans, ie 1 µM and 0.1 µM. No significant difference was observed in the effect of the two sartans. -

Angiotensin II Receptor Antagonists (Arbs), Renin
Angiotensin II Receptor Antagonists (ARBs), Renin Inhibitors, and Combinations Step Therapy Program Summary This program applies to Commercial, GenPlus, NetResults A series, Blue Partner, SourceRx, and Health Insurance Marketplace formularies. OBJECTIVE The intent of the Angiotensin II Receptor Antagonists (ARBs), Renin Inhibitors, and Combinations Step Therapy (ST) program is to encourage use of cost-effective generic products - ACEIs, ACEI combinations (ACEI/diuretics or ACEI/calcium channel blockers [CCBs]), ARBs, or ARB combinations - over the more expensive brand ARBs, brand ARB combinations, brand renin inhibitors and renin inhibitor combinations (renin inhibitor/diuretic, renin inhibitor/ARB, or renin inhibitor/CCB). This program will accommodate for use of brand products when generic prerequisites cannot be used due to previous trial and failure, documented intolerance, FDA labeled contraindication, or hypersensitivity. Requests for brand ARB or renin inhibitor products will be reviewed when patient-specific documentation is provided. TARGET AGENTS Angiotensin II Receptor Antagonists (ARBs), Combinations Brand Generic Atacand® candesartana Atacand HCT® candesartan/HCTZab Avapro® irbesartana Avalide® irbesartan/HCTZab Azor® olmesartan/amlodipinea Benicar® olmesartana Benicar HCT® olmesartan/HCTZab Byvalson™ nebivolol/valsartan Cozaar® losartana Diovan® valsartana Diovan HCT® valsartan/HCTZab Edarbi® azilsartan Edarbyclor® azilsartan/chlorthalidone Eprosartan eprosartan Exforge® valsartan/amlodipinea Exforge HCT® valsartan/amlodipine/HCTZab -

Free PDF Download
European Review for Medical and Pharmacological Sciences 2013; 17: 410-419 Pattern analysis and variations in the utilization of antihypertensive drugs in Taiwan: a six-year study L.-Y. HUANG1,2, W.-Y. SHAU1, H.-C. CHEN3, S. SU2, M.-C. YANG2, H.-L. YEH4,5, M.-S. LAI3,6 1Division of Health Technology Assessment, Center for Drug Evaluation, Taipei, Taiwan 2Graduate Institute of Health Care Organization Administration, College of Public Health, National Taiwan University, Taipei, Taiwan 3Graduate Institute of Epidemiology and Preventive Medicine, College of Public Health, National Taiwan University, Taipei, Taiwan 4Department of Cardiology, Sijhih Cathay General Hospital, Taipei, Taiwan 5School of Public Health, College of Public Health and Nutrition, Taipei Medical University, Taipei, Taiwan 6Center for Comparative Effectiveness Research, National Clinical Trial and Research Center, National Taiwan University Hospital, Taipei, Taiwan Abstract. – BACKGROUND: In the last few CONCLUSIONS: The consumption of antihy- years there have been changed in the pattern of pertension drugs in Taiwan increased during the consumption of antihypertensive drugs in other period studied and the highest average annual countries. Factors causing this variability in- increases were for ARBs and CCBs. Overall con- clude differences in the effectiveness of detec- sumption of antihypertension drugs also in- tion, guidelines for the management of hyperten- creased in other countries, but differences in the sion, and differences in national health insur- relative increase for each class of drug suggest ance systems among countries. that further study may be required to clarify the AIM: The aim of this study was to reveal pat- origins and causes. terns in the use of antihypertensive drugs in Tai- wan over a six year period (2001 to 2006) and com- Key Words: pare these results with data from other countries.