Candesartan Cilexetil – Atacand , Astra Merck Development And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Several Weeks Before Your Surgery, You Should Stop Taking
Several weeks before your surgery, you should stop taking: All Herbal/Vitamin Supplements & Weight loss medicines One week prior to surgery, you should stop taking: Aspirin products and Anti-inflammatory products (OTC and Prescribed) Anticoagulants or blood thinners such as Coumadin, Pradaxa, Plavix, Ticlid, and Aggrenox Night before surgery: Do not drink alcoholic beverages 24 hours prior to surgery. Alcohol may increase the depth of your anesthesia or the effect of medicines you may be given. Do not smoke after midnight prior to having surgery. Smoking can cause gastric secretions that interfere with the anesthetic during surgery. You need to be fasting or nothing to eat or drink 9 hours prior to your surgery. If your surgery is before noon, nothing to eat or drink after 11pm. If your surgery is after noon, nine hours prior to surgery you may eat a light meal (toast with clear juices such as apple juice or ginger ale). Here are some other pre-surgery instructions you need to follow: Do not take the following Blood pressure medications the night before or the morning of surgery: o Do not take ACE Inhibitors : Benazepril (Lotensin), Captopril (Capoten), Enalapril (Vasotec), Fosinpril (Monopril), Lisinopril (Prinvil, Zestril), Moexipril (Univasc or Perdix), Perindopril (Aceon), Quinapril (Accupril), Ramipril (Altace), Trandolapril (Mavik). o Do not take ARBS (Angiotenensin II Receptor Blockers): (Atacand) Candesartan, (Avapro) Irbesartan, (Benicar) Olmesartan, (Cozaar) Losartan, (Diovan) Valsartan, (Micardis) Telmisartan, (Teveten) Eprosartan The morning of surgery when you wake, take the following medications with a small sip of water: o Parkinson’s and seizure medications, blood pressure and heart medication except as indicated above.* o Do not take your diuretics or “water pills” the morning of surgery. -

Key Features of Candesartan Cilexetil and a Comparison with Other Angiotensin II Receptor Antagonists
Journal of Human Hypertension (1999) 13, (Suppl 1), S3–S10 1999 Stockton Press. All rights reserved 0950-9240/99 $12.00 Key features of candesartan cilexetil and a comparison with other angiotensin II receptor antagonists PS Sever Imperial College of Science, Technology & Medicine at St Mary’s Hospital, London, UK Current research on angiotensin II AT1-receptor antag- in patients with essential hypertension. Candesartan onists (AIIRAs) and selected studies presented at the cilexetil has a rapid onset of action (approximately 80% recent symposium held in Amsterdam, The Netherlands, of total blood pressure reduction within the first 2 on 6 June 1998, titled ‘Angiotensin II Receptor Antagon- weeks) and dose-dependent effects on blood pressure, ists are NOT all the Same’ are reviewed. AIIRAs offer a is comparable in efficacy to a number of classes of anti- number of potential advantages over alternative antihy- hypertensives, and is effective in combination therapy pertensive agents acting via the renin-angiotensin-aldo- (eg, with hydrochlorothiazide and amlodipine). This sterone system. They combine blood pressure-lowering favourable profile may be due in part to the highly selec- effects at least equivalent to those of angiotensin-con- tive, tight binding to and slow dissociation of candesar- verting enzyme (ACE) inhibitors, coupled with placebo- tan from the AT1 receptor. Preliminary studies suggest like tolerability. Candesartan cilexetil is a novel AIIRA that candesartan cilexetil also protects end organs that has demonstrated clinical -

Moexipril Hydrochloride | Medchemexpress
Inhibitors Product Data Sheet Moexipril hydrochloride • Agonists Cat. No.: HY-B0378A CAS No.: 82586-52-5 Molecular Formula: C₂₇H₃₅ClN₂O₇ • Molecular Weight: 535.03 Screening Libraries Target: Angiotensin-converting Enzyme (ACE); Apoptosis Pathway: Metabolic Enzyme/Protease; Apoptosis Storage: Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month SOLVENT & SOLUBILITY In Vitro DMSO : 250 mg/mL (467.26 mM; Need ultrasonic) H2O : 100 mg/mL (186.91 mM; Need ultrasonic) Mass Solvent 1 mg 5 mg 10 mg Concentration Preparing 1 mM 1.8691 mL 9.3453 mL 18.6905 mL Stock Solutions 5 mM 0.3738 mL 1.8691 mL 3.7381 mL 10 mM 0.1869 mL 0.9345 mL 1.8691 mL Please refer to the solubility information to select the appropriate solvent. In Vivo 1. Add each solvent one by one: 10% DMSO >> 40% PEG300 >> 5% Tween-80 >> 45% saline Solubility: ≥ 2.08 mg/mL (3.89 mM); Clear solution 2. Add each solvent one by one: 10% DMSO >> 90% (20% SBE-β-CD in saline) Solubility: ≥ 2.08 mg/mL (3.89 mM); Clear solution 3. Add each solvent one by one: 10% DMSO >> 90% corn oil Solubility: ≥ 2.08 mg/mL (3.89 mM); Clear solution BIOLOGICAL ACTIVITY Description Moexipril hydrochloride is a potent orally active non-sulfhydryl angiotensin converting enzyme(ACE) inhibitor, which is used for the treatment of hypertension and congestive heart failure. Target: ACEMoexipril hydrochloride is a long-acting ACE inhibitor suitable for once-daily administration, and like some ACE inhibitors, moexipril is a prodrug and needs to be hydrolyzed in the liver into its active carboxylic metabolite, moexiprilat, to become effective [1]. -

Download Leaflet View the Patient Leaflet in PDF Format
Package leaflet: Information for the patient Fosinopril Sodium 10 mg Tablets Fosinopril Sodium 20 mg Tablets Fosinopril sodium Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. - Keep this leaflet. You may need to read it again. - If you have any further questions, ask your doctor or pharmacist. - This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. - If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet 1. What Fosinopril is and what it is used for 2. What you need to know before you take Fosinopril 3. How to take Fosinopril 4. Possible side effects 5. How to store Fosinopril 6. Contents of the pack and other information. 1. What Fosinopril is and what is it used for Fosinopril belongs to the class of medicines called Angiotensin Converting Enzyme (ACE) inhibitors that act on the heart and blood vessels. You may have been given Fosinopril to: • lower your blood pressure if it is too high (a condition called hypertension) • help your heart pump blood around your body if you have a condition known as heart failure and are also being treated with diuretics (medicines which help to remove excess fluid from the body). 2. What you need to know before you take Fosinopril Do not take Fosinopril: • if you are allergic to fosinopril or any other ACE inhibitor, or any of the other ingredients of this medicine (listed in section 6). -

Ideal Drug for Blood Pressure
Int. J. Pharm. Sci. Rev. Res., 25(2), Mar – Apr 2014; Article No. 02, Pages: 7-12 ISSN 0976 – 044X Research Article Ideal Drug for Blood Pressure D. Praba1, M. Menakha2, K.A.Jeyanthi3 1Department of Biotechnology, Thanthai Hans Roever College of arts and science, Perambalur district, Tamil Nadu, India. 2P.G. and Research Department of Botany and Microbiology, A.V.V.M. Sri Pushpam College (Autonomous), Poondi, Thanjavur district, Tamil Nadu, India. 3Department of Biochemistry, Thanthai Hans Roever College of arts and science, Perambalur district, Tamil Nadu, India. *Corresponding author’s E-mail: [email protected] Accepted on: 27-11-2013; Finalized on: 31-03-2014. ABSTRACT Blood pressure, sometimes referred to as arterial blood pressure, is the pressure exerted by circulating blood upon the walls of blood vessels. Angiotensin is a peptide hormone that causes vasoconstriction followed by increase in blood pressure. Angiotensin I is converted to angiotensin II by the enzyme angiotensin-converting enzyme (ACE). ACE is a target for inactivation by ACE inhibitor drugs. ACE inhibitors are major anti-hypertensive drugs. Here, the ACE is disease causing receptor and the ACE inhibitors used as ligand. In the present investigation, most suitable ACE inhibitor was identified from frequently prescribed drugs such as perindopril, captopril, enalapril, lisinopril, ramipril, benazepril, fosinopril, trandolapril, moexipril and telmisartan through molecular docking. Based on the precision rate of docking and e-values, Trandolapril, Ramipril and Benazepril were identified as effective drug molecules. From the present investigation it is suggested that Trandolapril, Ramipril and Benazepril drug molecules could be used for regulating the blood pressure (Hypertension). -
Download Leaflet View the Patient Leaflet in PDF Format
Package leaflet: Information for the user Candesartan cilexetil 2 mg tablets Candesartan cilexetil 4 mg tablets Candesartan cilexetil 8 mg tablets Candesartan cilexetil 16 mg tablets Candesartan cilexetil 32 mg tablets candesartan cilexetil Read all of this leaflet carefully If you are going to have an operation, before you start taking this tell your doctor or dentist that you are medicine because it contains taking Candesartan cilexetil. This is important information for you. because Candesartan cilexetil, when - Keep this leaflet. You may need combined with some anaesthetics, to read it again. may cause an excessive drop in - If you have any further questions, blood pressure. ask your doctor or pharmacist. - This medicine has been Children and adolescents prescribed for you only. Do not Candesartan Cilexetil has been pass it on to others. It may harm studied in children. For more them, even if their signs of illness information, talk to your doctor. are the same as yours. Candesartan Cilexetil must not be - If you get any side effects, talk to given to children under 1 year of your doctor or pharmacist. This age due to the potential risk to the includes any possible side effects developing kidneys. not listed in this leaflet. See Other medicines and Candesartan section 4. cilexetil What is in this leaflet Tell your doctor or pharmacist if you 1. What Candesartan cilexetil is and are taking, have recently taken or what it is used for might take any other medicines. 2. What you need to know before you take Candesartan cilexetil Candesartan cilexetil can affect the 3. -

Angiotensin-Converting Enzyme Inhibition but Not Angiotensin II Receptor Blockade Regulates Matrix Metalloproteinase Activity in Patients with Glomerulonephritis
J Am Soc Nephrol 14: 2861–2872, 2003 Angiotensin-Converting Enzyme Inhibition but not Angiotensin II Receptor Blockade Regulates Matrix Metalloproteinase Activity in Patients with Glomerulonephritis NADE` GE LODS,* PAOLO FERRARI,* FELIX J. FREY,* ANDREAS KAPPELER,† CELINE BERTHIER,* BRUNO VOGT,* and HANS-PETER MARTI* *Division of Nephrology and Hypertension, Inselspital Bern, Bern, Switzerland; and †Institute of Pathology, University of Bern, Bern, Switzerland Abstract. Equivalent long-term effects on the kidney are attrib- periods of 4 wk each without therapy. Untreated patients with uted to angiotensin-converting enzyme inhibitors (ACEI) and glomerulonephritis displayed distinctively higher serum levels angiotensin II type 1 receptor blockers (ARB). Nevertheless, it of MMP-2 but much lower MMP-1/-8/-9 concentrations com- is unknown to which degree effects of these compounds on pared with healthy control subjects. Immunohistology of individual inflammatory mediators, including matrix metallo- MMP-2 and MMP-9 in kidney biopsy specimen was accord- proteinases (MMP), are comparable. On the basis of structural ingly. However, these patients excreted higher amounts of and functional differences, it was hypothesized that ACEI and MMP-2 and MMP-9 in urine than healthy control subjects, ARB differentially regulate MMP activity. In a randomized, possibly reflecting ongoing glomerular inflammation. In pa- prospective crossover trial, the effect of an ACEI (fosinopril; tients with glomerulonephritis, ACEI significantly reduced 20 mg/d) and of an ARB (irbesartan; 150 mg/d) on MMP overall MMP serum activity to 25%, whereas ARB did not activity was evaluated. Ten hypertensive patients with glomer- show any effect. Activities of MMP-1/-2/-8/-9 were also sig- ulonephritis and normal or mildly reduced creatinine clearance nificantly inhibited by fosinopril but not by irbesartan. -

Blocking the Tissue Renin-Angiotensin System: the Future Cornerstone of Therapy
Journal of Human Hypertension (2000) 14, Suppl 2, S23–S31 2000 Macmillan Publishers Ltd All rights reserved 0950-9240/00 $15.00 www.nature.com/jhh Blocking the tissue renin-angiotensin system: the future cornerstone of therapy T Unger1, M Azizi2 and GG Belz3 1Institute of Pharmacology, Christian Albrechts University, Hospitalstrae 4, 24105 Kiel, Germany; 2Centre D’Investigations Cliniques, Hoˆ pital Broussais, 96 Rue Didot – 75674, Paris Cedex 14, France; 3Zentrum fuer Kardiovaskulaere Pharmakologie, ZeKaPha GmbH, Mathildenstrae 8, D-55116, Mainz-Wiesbaden, Germany The development of angiotensin-converting enzyme antagonist, is characterised by its tight binding to and inhibitors and selective angiotensin type 1 (AT1)- slow dissociation from the AT1 receptor, and high antag- receptor antagonists has provided new insights into onistic potency, resulting in long-lasting antagonistic understanding the mechanism of the renin-angiotensin effects. It is anticipated that these pharmacological system (RAS) in the pathophysiology of cardiovascular characteristics may bring additional benefits to patients, disease. There is good evidence from meta-analyses not only for the management of essential hypertension that shows that inhibition of the RAS achieves organ but also for the management of end-organ damage. protection features that go beyond blood pressure con- Journal of Human Hypertension (2000) 14, Suppl 2, S23– trol. Candesartan cilexetil, a new angiotensin II receptor S31 Keywords: renin-angiotensin system; angiotensin-converting enzyme inhibitor; angiotensin type 1 receptor; angiotensin receptor antagonist; candesartan cilexetil Introduction Blocking the RAS in essential Hypertension is a major risk factor for myocardial hypertension infarction, stroke, and renal and peripheral vascular Angiotensin II, the key effector peptide of the RAS, disease. -
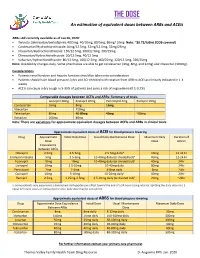
THE DOSE an Estimation of Equivalent Doses Between Arbs and Aceis
THE DOSE An estimation of equivalent doses between ARBs and ACEIs ARBs still currently available as of Jan 26, 2020: Twynsta (telmisartan/amlodipine): 40/5mg. 40/10mg, 80/5mg, 80mg/ 10mg Note: ~$0.73/tablet (ODB covered) Candesartan/Hydrochlorothiazide:16mg/12.5mg, 32mg/12.5mg, 32mg/25mg Irbesartan/Hydrochlorothiazide: 150/12.5mg, 300/12.5mg, 300/25mg Olmesartan/Hydrochlorothiaizde: 20/12.5mg, 40/12.5mg Valsartan/Hydrochlorothiazide: 80/12.5mg, 160/12.5mg, 160/25mg, 320/12.5mg, 320/25mg Note: Availability changes daily. Some pharmacies are able to get candesartan (4mg, 8mg, and 32mg) and irbesartan (300mg). Considerations Patients renal function and hepatic function should be taken into consideration Patients should have blood pressure, lytes and SCr checked with rotation from ARB to ACEI as clinically indicated in 1-4 weeks ACEIs can cause a dry cough in 5-35% of patients and carry a risk of angioedema (0.1-0.2%) Comparable dosages between ACEIs and ARBs- Summary of trials Lisinopril 20mg Enalapril 20mg Perindopril 4mg Ramipril 10mg Candesartan 16mg 8mg 16mg Irbesartan 150mg Telmisartan 80mg 40-80mg 40mg ~80mg Valsartan 160mg 80mg Note: There are variations for approximate equivalent dosages between ACEIs and ARBs in clinical trials. Approximate equivalent doses of ACEI for blood pressure lowering Drug Approximate Initial Daily Dose Usual Daily Maintenance Dose Maximum Daily Duration of Dose Dose Action Equivalence Between ACEIs Cilazapril 2.5mg 2.5-5mg 2.5-5mg dailya 10mg 12-24 hr Enalapril maleate 5mg 2.5-5mg 10-40mg daily (or divided bid)a 40mg 12-24 hr Fosinopril 10mg 10mg 10-40mg daily (or divided bid)a 40mg 24hr Lisinopril 10mg 2.5-10mg 10-40mg daily 80mg 24hr Perindopril 2mg 2-4mg 4-8mg daily 8mg 24hr Quinapril 10mg 5-10mg 10-20mg dailya 40mg 24hr Ramipril 2.5mg 1.25mg-2.5mg 2.5-10mg daily (or divided bid)a 20mg ~24hr a: Some patients may experience a diminished antihypertensive effect toward the end of a 24-hour dosing interval. -

Angiotensin-Converting Enzyme (ACE) Inhibitors
Angiotensin-Converting Enzyme (ACE) Inhibitors Summary Blood pressure reduction is similar for the ACE inhibitors class, with no clinically meaningful differences between agents. Side effects are infrequent with ACE inhibitors, and are usually mild in severity; the most commonly occurring include cough and hypotension. Captopril and lisinopril do not require hepatic conversion to active metabolites and may be preferred in patients with severe hepatic impairment. Captopril differs from other oral ACE inhibitors in its rapid onset and shorter duration of action, which requires it to be given 2-3 times per day; enalaprilat, an injectable ACE inhibitor also has a rapid onset and shorter duration of action. Pharmacology Angiotensin Converting Enzyme Inhibitors (ACE inhibitors) block the conversion of angiotensin I to angiotensin II through competitive inhibition of the angiotensin converting enzyme. Angiotensin is formed via the renin-angiotensin-aldosterone system (RAAS), an enzymatic cascade that leads to the proteolytic cleavage of angiotensin I by ACEs to angiotensin II. RAAS impacts cardiovascular, renal and adrenal functions via the regulation of systemic blood pressure and electrolyte and fluid balance. Reduction in plasma levels of angiotensin II, a potent vasoconstrictor and negative feedback mediator for renin activity, by ACE inhibitors leads to increased plasma renin activity and decreased blood pressure, vasopressin secretion, sympathetic activation and cell growth. Decreases in plasma angiotensin II levels also results in a reduction in aldosterone secretion, with a subsequent decrease in sodium and water retention.[51035][51036][50907][51037][24005] ACE is found in both the plasma and tissue, but the concentration appears to be greater in tissue (primarily vascular endothelial cells, but also present in other organs including the heart). -

Comparison of the Efficacy of Candesartan and Losartan: a Meta-Analysis of Trials in the Treatment of Hypertension
Journal of Human Hypertension (2010) 24, 525–531 & 2010 Macmillan Publishers Limited All rights reserved 0950-9240/10 www.nature.com/jhh ORIGINAL ARTICLE Comparison of the efficacy of candesartan and losartan: a meta-analysis of trials in the treatment of hypertension PA Meredith, LS Murray and GT McInnes Department of Medicine & Therapeutics, Division of Cardiovascular & Medical Sciences, Gardiner Institute, Western Infirmary, Glasgow, Scotland, UK Informed by the findings from prospective observational included in the analysis using a random effect model. studies and randomized outcome trials, guidelines for Mean changes in SBP and DBP were compared for each the management of hypertension acknowledge that drug alone and after stratification for dose and for the benefit of treatment can be attributed largely to combination with hydrochlorothiazide (HCTZ). On the blood pressure (BP) reduction. Therefore, quantification basis of all the data, the weighted mean difference of differential BP lowering of different agents within favoured candesartan—3.22 mm Hg (95% confidence classes of anti-hypertensives is of practical importance. interval (CI) 2.16, 4.29) for SBP and 2.21 mm Hg (95% CI The objective of this analysis was to compare the 1.34, 3.07) for DBP. These findings were consistent efficacy of candesartan and losartan with respect to when analyses according to dose and combination with reduction in systolic and diastolic BP (SBP and DBP). HCTZ were carried out. Thus, it can be concluded that at A systematic literature search of databases from 1980 to currently recommended doses, candesartan is more 1 October 2008 identified 13 studies in which candesartan effective than losartan in lowering BP. -

Antidepressant Activity of Fosinopril, Ramipril and Losartan, but Not of Lisinopril in Depressive Paradigms of Albino Rats and Mice
Indian Journal of Experimental Biology Vol. 46, March 2008, pp. 180-184 Antidepressant activity of fosinopril, ramipril and losartan, but not of lisinopril in depressive paradigms of albino rats and mice Veena Nayak & P A Patil* Department of Pharmacology & Pharmacotherapeutics, J.N.Medical College, Belgaum 590 010, India Received 15 January 2007; revised 15 January 2008 Fosinopril, ramipril and losartan significantly decreased the duration (sec) of immobility in forced swim test and were comparable to amitriptyline. The duration of immobility were significantly decreased in fosinopril, ramipril and losartan in the tail suspension test and were comparable to amitriptyline. Only losartan significantly increased the rearing number of entries, time spent (sec) in open arm and in light area in comparison to control animals. Fosinopril and ramipril and not lisinopril showed significant antidepressant activity while losartan showed a significant antidepressant and anxiolytic activity. Present findings suggest that these drugs could be better antihypertensives in hypertensive patients with co- morbidity like depression or anxiety. Keywords: Anxiety, Depression, Fosinopril, Lisinopril, Losartan, Ramipril Physiological depression and anxiety when become possess antidepressant and anxiolytic activity like severe and chronic lead to a variety of psychiatric captopril and enalapril. A report regarding quinapril13 disorders. Quite often such mood disorders could be indicates that all ACE inhibitors may not share secondary to a number of cardiovascular and antidepressant and anxiolytic activity and such endocrinal disorders1,2 requiring treatment with two activities are not probed with other ACE inhibitors drugs, one for physical or metabolic and the other for used clinically. It is therefore planned to investigate associated mental disorder.