Biosafety Site-Specific Training Checklist & Record
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Biological Safety Standard Operating Procedure (SOP) SOP Title
IBSP005 Biosafety SOP template January 2021 Icahn School of Medicine at Mount Sinai Biological Safety Standard Operating Procedure (SOP) SOP Title SOP Number (version) IBC registration(s) Section 1. Laboratory-specific information Building/Room(s) Department/Institute SOP author LSO PI name PI signature Section 2. Biological hazard information Biological Agent1 RG2 BSL3 Potential Hazards Signs/Symptoms of Occupational Health ABSL4 Infection Requirements Section 3. Other hazards Sharps Hazards Other Biological Hazards Chemical Hazards Radiological Hazards Section 4. Personal Protective Equipment (PPE) Laboratory Coat Fluid-Resistant Gown Surgical Mask Gloves Shoe Covers N95 Respirator Eye Protection Fluid-Resistant Sleeves PAPR Other PPE 1 Specific strains of biological agents should be described in the corresponding IBC registration. 2 NIH Risk Groups: RG-1, RG-2, RG-3, or RG-4 3 Biological Safety Level: BSL-1, BSL-2, BSL-3, BSL-4 4 Animal Biological Safety Level: ABSL-1, ABSL-2, ABSL-3, ABSL-4 Page | 1 IBSP005 Biosafety SOP template January 2021 Section 5. Equipment and Engineering Controls Biological Safety Cabinet Centrifuge Aerosol-Generating Equipment Other equipment Section 6. Decontamination Disinfectant Contact Time Dilution Location Section 7. Waste Management/Disposal Steam Sterilizer Location: (Autoclave) Chemical Disinfectant(s) Location: Other Disinfectant(s) Location: Section 8. Transport Procedure(s) Page | 2 IBSP005 Biosafety SOP template January 2021 Section 9. Spill Response Procedure Section 10. Protocol Procedure Page | 3 IBSP005 Biosafety SOP template January 2021 Section 10. Protocol Procedure Continued (attach additional sheets if necessary) Page | 4 IBSP005 Biosafety SOP template January 2021 Section 11. Documentation of training and understanding. The Principal Investigator must ensure that all laboratory personnel receive training on the content of this SOP. -

Occupational Safety and Health Admin., Labor § 1910.145
Occupational Safety and Health Admin., Labor § 1910.145 of the workers or their families, shall (i) Fire protection equipment and appa- be provided in connection with all food ratus. [Reserved] handling facilities. There shall be no (ii) Danger. Safety cans or other port- direct opening from living or sleeping able containers of flammable liquids quarters into a kitchen or dining hall. having a flash point at or below 80° F, (3) No person with any communicable table containers of flammable liquids disease shall be employed or permitted (open cup tester), excluding shipping to work in the preparation, cooking, containers, shall be painted red with serving, or other handling of food, food- some additional clearly visible identi- stuffs, or materials used therein, in fication either in the form of a yellow any kitchen or dining room operated in band around the can or the name of the connection with a camp or regularly contents conspicuously stenciled or used by persons living in a camp. painted on the can in yellow. Red (j) Insect and rodent control. Effective lights shall be provided at barricades measures shall be taken to prevent in- and at temporary obstructions, as spec- festation by and harborage of animal ified in ANSI Safety Code for Building or insect vectors or pests. Construction, A10.2±1944, which is in- (k) First aid. (1) Adequate first aid fa- corporated by reference as specified in cilities approved by a health authority § 1910.6. Danger signs shall be painted shall be maintained and made available red. in every labor camp for the emergency (iii) Stop. -
Lab Standard Operating Procedures (Sops)
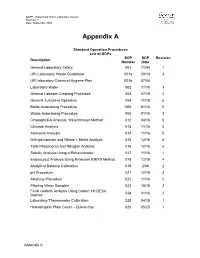
QAPP –Watershed Watch Laboratory Assays Revision: 7 Date: September 2020 Appendix A Standard Operation Procedures List of SOPs SOP SOP Revision Description Number Date General Laboratory Safety 001 11/04 1 URI Laboratory Waste Guidebook 001a 09/13 3 URI laboratory Chemical Hygiene Plan 001b 07/04 Laboratory Water 002 11/16 3 General Labware Cleaning Procedure 003 07/19 4 General Autoclave Operation 004 10/18 6 Bottle Autoclaving Procedure 005 01/16 5 Waste Autoclaving Procedure 006 01/16 3 Chlorophyll-A Analysis, Welschmeyer Method 012 04/18 6 Chloride Analysis 013 11/16 5 Ammonia Analysis 014 11/16 5 Orthophosphate and Nitrate + Nitrite Analysis 015 12/16 5 Total Phosphorus and Nitrogen Analysis 016 12/16 5 Salinity Analysis Using a Refractometer 017 11/16 1 Enterococci Analysis Using Enterolert IDEXX Method 018 12/19 4 Analytical Balance Calibration 019 2/06 2 pH Procedure 021 12/19 3 Alkalinity Procedure 022 11/16 2 Filtering Water Samples 023 10/18 2 Fecal coliform Analysis Using Colilert 18 IDEXX 024 01/16 2 Method Laboratory Thermometer Calibration 025 04/19 1 Heterotrophic Plate Count – Quanti-tray 026 05/20 1 Appendix A Standard Operating Procedure 001 Date: 11/04 General Laboratory Safety Revision: 1 Author: Linda Green University of Rhode Island Watershed Watch 1.0 PURPOSE AND DESCRIPTION LAB SAFETY IS EVERYBODY’S JOB! Please be sure to familiarize yourself with these general procedures, as well as the specific handling requirements included in the Standard Operating Procedure (SOP) for each analysis/process. Further general information regarding University of Rhode Island standards for health and safety are found in SOP 001a – University Safety and Waste Handling Document. -
Sampling Guide for First Responders to Drinking Water Contamination Threats and Incidents
Sampling Guide for First Responders to Drinking Water Contamination Threats and Incidents This First Responders Sampling Guide was funded under U.S. EPA Assistance Agreement No. X6-97109101-1. Sampling Guide for First Responders to Drinking Water Contamination Threats and Incidents New England Water Works Association 125 Hopping Brook Road Holliston, MA 01746-1471 November 2008 Acknowledgments This Sampling Guide for First Responders to Drinking Water Contamination Threats and Incidents was originally developed by the New England Water Works Association in collaboration with U.S. EPA. Please refer to pages 75 and 76 for a complete list of acknowledgments. The cover photo is reprinted with permission from the Worcester Telegram & Gazette. IMPORTANT NOTICE The methods and instructions in this guide reflect the U.S. Environmental Protection Agency (EPA) regulations and guidance, in addition to other reference documents. It is recognized that there can be significant differences from state to state regarding public notification, sampling procedures, laboratory safety, and handling, etc. Please check with your state drinking water representative with any questions before First Responders training and preparation is begun. Contents, Sections 1 through 6 1. Introduction 1.1 Background/Purpose ......................................1 1.2 How to Use This Guide ....................................2 2. Overview of Site Characterization .........................3 2.1 Investigating the Site .......................................3 2.2 Who Conducts Site Characterization and Sampling? .................................................5 3. Site Characterization Five-Step Process ...............6 3.1 The Five-Step Process .....................................6 Step 1: Customizing the Site Characterization Plan ........................8 Step 2: Approaching the Site and Doing a Field Safety Screening ........9 Step 3: Characterizing the Site ...................11 Step 4: Collecting Samples ..........................14 Step 5: Exiting the Site .................................16 4. -

Commodity Specific Food Safety Guidelines for the Fresh Tomato Supply Chain
COMMODITY SPECIFIC FOOD SAFETY GUIDELINES FOR THE FRESH TOMATO SUPPLY CHAIN Third Edition | September 2018 Commodity Specific Food Safety Guidelines for the Fresh Tomato Supply Chain Tomato Guidelines, 3rd Edition User’s Note These guidelines provide recommended food safety practices that are intended to minimize the microbiological hazards associated with fresh and fresh-cut tomato products. The intent of drafting this document is to provide currently available information on food safety and handling in a manner consistent with existing applicable regulations, standards and guidelines. The information provided herein is offered in good faith and believed to be reliable, but is made without warranty, express or implied, as to merchantability, fitness for a particular purpose, or any other matter. These recommended guidelines were not designed to apply to any specific operation. It is the responsibility of the user of this document to verify that these guidelines are appropriate for its operation. The publishing trade associations, their members and contributors do not assume any responsibility for compliance with applicable laws and regulations, and recommend that users consult with their own legal and technical advisers to be sure that their own procedures meet with applicable requirements. - 1 - Foreword The North American Tomato Trade Work Group (NATTWG) published in 2006 the first edition of Commodity Specific Food Safety Guidelines for the Fresh Tomato Supply Chain (“Guidelines”). Within two years of publication, several initiatives resulted in significant new learnings about potential risks and control measures at all points in the fresh tomato supply chain. Some of those initiatives include the FDA Tomato Safety Initiative, voluntary efforts by the Florida Tomato Exchange and the California Tomato Farmers to develop USDA-verified audit criteria and programs for tomato production and harvest practices in those states, and several retail and foodservice buyer initiatives to further define tomato safe growing and handling practices. -

Finding Hazards
FINDING HAZARDS OSHA 11 Finding Hazards 1 Osha 11 Finding Hazards 2 FINDING HAZARDS Learning Objectives By the end of this lesson, students will be able to: • Define the term “job hazard” • Identify a variety of health and safety hazards found at typical worksites where young people are employed. • Locate various types of hazards in an actual workplace. Time Needed: 45 Minutes Materials Needed • Flipchart Paper • Markers (5 colors per student group) • PowerPoint Slides: #1: Job Hazards #2: Sample Hazard Map #3: Finding Hazards: Key Points • Appendix A handouts (Optional) Preparing To Teach This Lesson Before you present this lesson: 1. Obtain a flipchart and markers or use a chalkboard and chalk. 2. Locate slides #1-3 on your CD and review them. If necessary, copy onto transparencies. 3. For the Hazard Mapping activity, you will need flipchart paper and a set of five colored markers (black, red, green, blue, orange) for each small group. Detailed Instructor’s Notes A. Introduction: What is a job hazard? (15 minutes) 1. Remind the class that a job hazard is anything at work that can hurt you, either physically or mentally. Explain that some job hazards are very obvious, but others are not. In order to be better prepared to be safe on the job, it is necessary to be able to identify different types of hazards. Tell the class that hazards can be divided into four categories. Write the categories across the top of a piece of flipchart paper and show PowerPoint Slide #1, Job Hazards. • Safety hazards can cause immediate accidents and injuries. -

Working with Biological Agents (PDF, 564KB)
Working with biological agents Guidance notes These Notes provide guidance on aspects of work with biological agents. Several contain MRC Codes of Practice (CoP). Those containing a CoP are marked with * in the index below. Where a CoP is specified, establishments are expected to achieve and maintain those standards, which are set as the minimum acceptable for carrying out the related work. The associated guidance will aid the achievement of MRC standards and ensure the work meets all related legal requirements. In all MRC CoPs, 'must' indicates a mandatory requirement whereas 'should' reflects a strong recommendation. Statements using should relate to the required standard and any modification must be justified through the process of a suitable and sufficient risk assessment. Where appropriate the content of these notes can be adopted as local codes of practice and referred to by risk assessments. Establishments are encouraged however to adapt the content for local needs, with the strict provision that local standards are at least as high as those stipulated in this guidance. Part A The management of work with biological material Guidance note 1 a) The appointment and duties of a Biological Safety Officer: b) The formation and remit of Biological Safety Committees Guidance note 2 Laboratory management Guidance note 3 Risk assessments and related procedures - overview Guidance note 4 Use of blood from volunteer staff, including use of ‘own’ cells* Part B Working with biological hazards Guidance note 5 Working with human pathogens* Guidance note 6 Working with transmissible spongiform encephalopathies* Guidance note 7 Working with genetically modified organisms Guidance Note 8 Working with cell cultures Guidance Note 9 Working with human material: 1. -

Personal Protective Equipment
LABORATORY BIOSAFETY MANUAL FOURTH EDITION AND ASSOCIATED MONOGRAPHS PERSONAL PROTECTIVE EQUIPMENT LABORATORY BIOSAFETY MANUAL FOURTH EDITION AND ASSOCIATED MONOGRAPHS PERSONAL PROTECTIVE EQUIPMENT Personal protective equipment (Laboratory biosafety manual, fourth edition and associated monographs) ISBN 978-92-4-001141-0 (electronic version) ISBN 978-92-4-001142-7 (print version) © World Health Organization 2020 Some rights reserved. This work is available under the Creative Commons Attribution- NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/ licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization (http://www.wipo.int/amc/en/ mediation/rules/). Suggested citation. Personal protective equipment. Geneva: World Health Organization; 2020 (Laboratory biosafety manual, fourth edition and associated monographs). -

Complete Manual Cdc-Pdf
Framework for Assessing Health Impacts of Multiple Chemicals and Other Stressors (Update) U.S. Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry Division of Toxicology February 2018 ii PREFACE The mission of the Agency for Toxic Substances and Disease Registry (ATSDR) is to serve the public by using the best science, taking responsive public health actions, and providing trusted health information to prevent harmful exposures and disease related to toxic substances. The U.S. Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA, also known as the Superfund act) mandates that ATSDR determine whether people living near or at a hazardous waste site are being exposed, have been exposed, or will be exposed to toxic substances, whether that exposure is harmful, and what can be done to stop or reduce harmful exposures. CERCLA requires that ATSDR consider the following factors when evaluating the possible public health impacts of communities near Superfund sites: (1) the nature and extent of contamination at a site; (2) the demographics of the site population; (3) exposure pathways that may exist at a site (the extent to which people contact site contaminants); and (4) health effects and disease-related data. In addition, ATSDR is also authorized to conduct public health assessments at storage, treatment, and disposal facilities for hazardous wastes when requested by EPA, under the 1984 amendments to the Resource Conservation and Recovery Act of 1976 (RCRA). In addition, ATSDR conducts public health assessments for toxic substances, when petitioned by concerned community members, physicians, state or federal agencies, or tribal governments. -

Exposure to Occupational Hazards Among Health Care Workers in Low- and Middle-Income Countries: a Scoping Review
International Journal of Environmental Research and Public Health Review Exposure to Occupational Hazards among Health Care Workers in Low- and Middle-Income Countries: A Scoping Review Rajni Rai 1,*, Sonia El-Zaemey 1, Nidup Dorji 2 , Bir Doj Rai 3 and Lin Fritschi 1 1 School of Public Health, Curtin University, Bentley, WA 6102, Australia; [email protected] (S.E.-Z.); [email protected] (L.F.) 2 Faculty of Nursing and Public Health, Khesar Gyalpo University of Medical Sciences of Bhutan, Thimphu 11001, Bhutan; [email protected] 3 Regional Livestock Development Centre Wangduephodrang, Wangduephodrang 14001, Bhutan; [email protected] * Correspondence: [email protected]; Tel.: +61-4-16090550 Abstract: Health care workers are exposed to numerous workplace hazards. The implementation of safety measures in high-income countries has largely mitigated these risks. However, in many low- and middle- income countries (LMICs), resources to institute safety measures are lacking, increasing the risk of occupational exposures to these hazards. The aim of this scoping review is to map and synthesize the available research on occupational hazards among health care workers in LMICs, identify research gaps and inform policy. Searches for relevant articles were conducted in five electronic databases using a broad range of search terms. The inclusion criteria were: quantitative observational or experimental studies which examined exposure to one or more occupational hazards among health care workers in a LMCI; and the article was published in English in a peer-reviewed journal. A total of 99 studies met the inclusion criteria, and data were extracted from these studies. -

Chapter 6 Biological Hazards
BIOLOGICAL HAZARDS TABLE OF CONTENTS Basic Informations Teacher’s Notes A. Hazard Identification and Control Review . 2 B. Biological Hazards Overview. .3 C. Types of Biological Hazards . 4 D. How Do Biological Hazards Enter the Body? . .6 E. How Biological Hazards are Spread . 8 F. Controlling Exposure to Biological Hazards . 11 • Engineering Controls . 11 • Administrative Controls . 12 • Personal Protective Equipment. 13 • Standard Precautions . 14 • WHMIS . 16 G. Pandemics. 17 Handout . 21 Presentations . 23 Supplemental Information Teacher’s Notes A. Agriculture and Landscaping . 35 Presentation. 37 © Government of Alberta Work Safe Alberta Occupational Health and Safety Teacher Resources 1 BIOLOGICAL HAZARDS Basic information TEACHER’S NOTES A. Hazard Identification and Control Review Detailed information on the legislated requirements for hazard assessment is included in the chapter on legislation. Detailed information on the process for identifying and controlling hazards and sample hazard assessment forms are included in the chapter on health and safety management systems. A brief overview is provided here to reinforce the importance of the basic principles of hazard identification, assessment and control. What is a Hazard? A hazard is any situation, condition or thing that may be dangerous to the safety or health of workers. (OHS Code Part 1) Identifying Hazards The first step in preventing incidents, injuries or illness in the workplace is identification of all the hazards within the workplace that could cause Where’s the hazard? injury or illness. In Alberta, the employer is responsible for conducting an overall hazard assessment in the workplace (OHS Code, Part 2); however, all workers should be able to recognize and identify hazards in the workplace on an ongoing basis. -

HSP - 407 Current Rev: 3/10/2017 Revised By: B
Document Number: HSP - 407 Current Rev: 3/10/2017 Revised By: B. Ayres Review Cycle: 3 years Manager Approval: H. Garner Revision No. 6 Page 1 of 17 HEALTH AND SAFETY PROGRAM 407 – BIOLOGICAL HAZARDS CONTROL PROGRAM 1.0 PURPOSE Westlake, Plaquemine Operations, has developed this program to minimize potential illnesses resulting from contact with biological agents, and enhance employee protection. The Biological Hazards Control Program establishes criteria to enhance employee awareness and training on the subjects of biological hazards (biohazards). Biohazards can be transmitted to employees through inhalation, injection, ingestion, or contact with the skin. Contact with biohazards can be minimized using a combination of engineering and work practice controls, personal protective equipment (PPE), training, medical surveillance, signs and labels. 2.0 SCOPE This program applies to all Westlake, Plaquemine Operations’ employees. 3.0 DEFINITIONS Biohazard Control--Any set of equipment, personal protective equipment, and procedures utilized to prevent or minimize the contact of employees and their environment to biohazardous agents or materials. Biological Hazards (Biohazards)--Biological agents or substances present in or arising from the work environment that present or may present a hazard to the health or well being of the worker or community. Potential biological agents or substances which could be biohazards include, but are not limited to, infectious or parasitic agents; infectious microorganisms such as some fungi; yeasts and algae; plants and plant products; and animals and animal products which may cause occupational disease. Biological Hazardous Waste--Any substance of human or animal origin, other than food wastes, which are to be disposed of, and could harbor or transmit pathogenic organisms including, but not limited to, pathological specimens such as tissues, blood elements, excreta, secretions, and related substances.