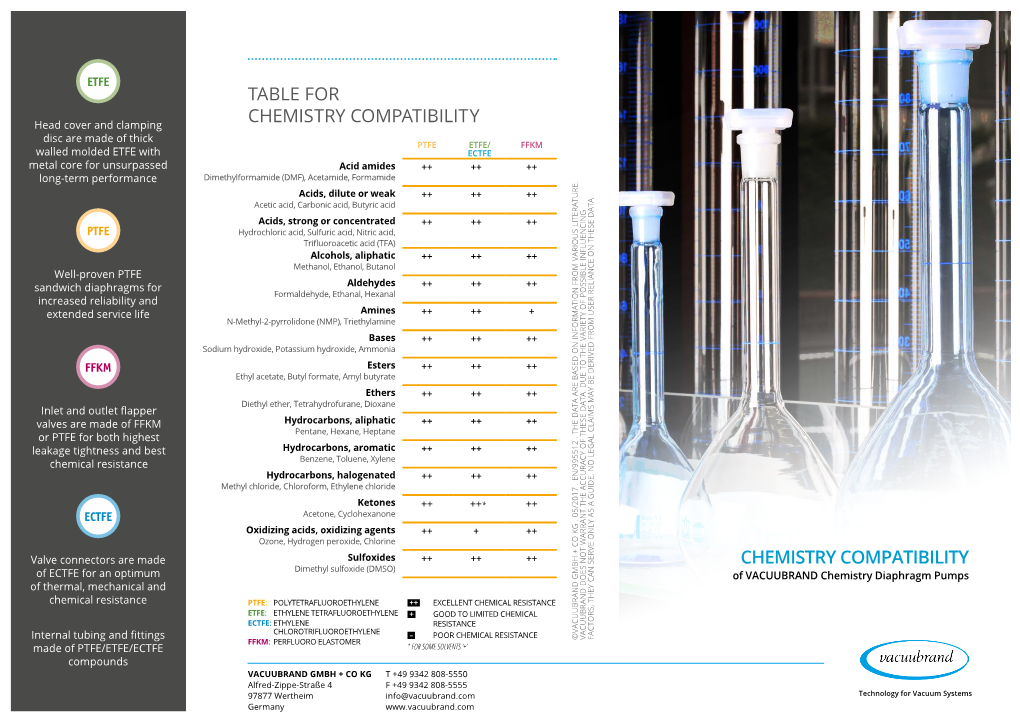
Table for Chemistry Compatibility
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Problem Formulation of the Risk Evaluation for Perchloroethylene (Ethene, 1,1,2,2-Tetrachloro)
EPA Document# EPA-740-R1-7017 May 2018 DRAFTUnited States Office of Chemical Safety and Environmental Protection Agency Pollution Prevention Problem Formulation of the Risk Evaluation for Perchloroethylene (Ethene, 1,1,2,2-Tetrachloro) CASRN: 127-18-4 May 2018 TABLE OF CONTENTS ABBREVIATIONS ............................................................................................................................ 8 EXECUTIVE SUMMARY .............................................................................................................. 11 1 INTRODUCTION .................................................................................................................... 14 1.1 Regulatory History ..................................................................................................................... 16 1.2 Assessment History .................................................................................................................... 16 1.3 Data and Information Collection ................................................................................................ 18 1.4 Data Screening During Problem Formulation ............................................................................ 19 2 PROBLEM FORMULATION ................................................................................................. 20 2.1 Physical and Chemical Properties .............................................................................................. 20 2.2 Conditions of Use ...................................................................................................................... -

Fluorinated Polymers As Smart Materials for Advanced Biomedical Applications
polymers Review Fluorinated Polymers as Smart Materials for Advanced Biomedical Applications Vanessa F. Cardoso 1,2,* ID , Daniela M. Correia 3,4, Clarisse Ribeiro 1,5 ID , Margarida M. Fernandes 1,5 and Senentxu Lanceros-Méndez 4,6 1 Centro/Departamento de Física, Universidade do Minho, 4710-057 Braga, Portugal; cribeiro@fisica.uminho.pt (C.R.); margaridafernandes@fisica.uminho.pt (M.M.F.) 2 CMEMS-UMinho, Universidade do Minho, DEI, 4800-058 Guimaraes, Portugal 3 Departamento de Química e CQ-VR, Universidade de Trás-os-Montes e Alto Douro, 5001-801 Vila Real, Portugal; [email protected] 4 BCMaterials, Basque Center for Materials, Applications and Nanostructures, UPV/EHU Science Park, 48940 Leioa, Spain; [email protected] 5 CEB—Centre of Biological Engineering, University of Minho, 4710-057 Braga, Portugal 6 IKERBASQUE, Basque Foundation for Science, 48013 Bilbao, Spain * Correspondence: [email protected]; Tel.: +351-253-60-40-73 Received: 11 January 2018; Accepted: 6 February 2018; Published: 8 February 2018 Abstract: Fluorinated polymers constitute a unique class of materials that exhibit a combination of suitable properties for a wide range of applications, which mainly arise from their outstanding chemical resistance, thermal stability, low friction coefficients and electrical properties. Furthermore, those presenting stimuli-responsive properties have found widespread industrial and commercial applications, based on their ability to change in a controlled fashion one or more of their physicochemical properties, in response to single or multiple external stimuli such as light, temperature, electrical and magnetic fields, pH and/or biological signals. In particular, some fluorinated polymers have been intensively investigated and applied due to their piezoelectric, pyroelectric and ferroelectric properties in biomedical applications including controlled drug delivery systems, tissue engineering, microfluidic and artificial muscle actuators, among others. -

ECTFE Data Sheet
ECTFE Fluoropolymer Extruded Films ETHYLENE-CHLOROTRIFLUOROETHYLENE FILM FOR USE IN HIGH-PERFORMANCE APPLICATIONS TCI’s ECTFE films are produced from HALAR® Ethylene-ChloroTrifluoroEthylene resins by a melt extrusion casting process. These films have the typical fluoropolymer traits of being resistant to harsh thermal, chemical, and ultraviolet environments. TCI’s ECTFE films offer excellent weatherability, chemical abrasion resistance, tear resistance, non-stick properties, and resistance to high-energy radiation. ECTFE films can be heat-sealed, thermoformed, and laminated to various substrates. TCI’s ECTFE films are utilized in a variety of industries: Chemical Processing TCI’s ECTFE Films Characteristics: Due to its superior chemical resistance to most acids and solvents over a broad temperature range Highest abrasion and tear resistance among all fluoropolymer films and low permeability to solvents and gases, ECTFE films applications include: chemical tank linings, pump Outstanding weatherability and resistance to diaphragms, chlorine cells, water treatment, spray UV radiation shielding applications for pipe joints, and semi- Chemically inert conductor processing environments. Excellent fire resistance, UL V-0 rating Outdoor Protection Superior anti-stick and low friction properties Excellent weatherability, non-stick properties, U-V Highest dielectric strength of all fluoropolymer stability, abrasion resistance, tear resistance, and high films light transmission make ECTFE film very effective for outdoor protective applications. Thermoformable and heat-sealable Continuous service temperature from Photovoltaic Panels -200°C (-328°F) up to 165°C (330°F) TCI’s ECTFE films offer excellent dielectric perfor- mance, superior water vapor barrier properties, fire resistance, and high solar transmittance. These films TCI’s ECTFE Films General Availability: are ideally suited for use in the back sheet and front Thickness range from 0.0005" to 0.010" sheet glazing of PV panels. -

Chemical Resistance
Materials - Chemical Resistance Please note: Substances All information in our catalogue is based on current technical knowledge, Substance Conc. PTFE PFA FEP ETFE ECTFE PVDF PP PA PS PMMA experience and manufacturers’ data. Users should check the suitability of at +20 °C in % parts and materials described in the catalogue before purchase. Accumulator acid 20 + + + + + + + – + – Acetaldehyde 100 + + + + + + ° –– ° BOLA does not accept any warranty claims as to suitability and fitness of Acetamide 100 + + + + + + + + + ° purpose of the materials and products described in this catalogue. Users Acetic acid 100 + + + + + + + – ° – should avoid making any assumptions on, or interpretation of, the data Acetic acid amide 100 + + + + + + + + + ° herein. Therefore we cannot provide warranty and cannot accept Acetic acid anhydride 100 + + + + + – ° ––– responsibility for any damage. Acetic acid butyl ester 100 + + + + + + ° + –– Acetic acid chloride 100 + + + + + + ° ° –– Acetic acid ethyl ester 100 + + + + + – ° + –– Acetic acid pentyl ester 100 + + + + + + + + – + Acetic anhydride 100 + + + + + – ° ––– Acetone 100 + + + + + – + + –– Acetonitrile 100 + + + + + ° + + –– Acetophenone 100 + + + + + + + ––– Acetyl benzene 100 + + + + + + + ––– Acetyl chloride 100 + + + + + + ° ° –– Acetylene tetrachloride 100 + + + –– + – + –– Acetylsalicylic acid 100 + + + + + + + + + – Acetone-2 100 + + + + + – + + –– Acrylic acid butyl ester 100 + + + + + ° ° + –– Acrylic acid ethylic ester 100 + + + + + ° ° + –– Acrylonitrile 100 + + + + + ° ° + –– Adipic acid -

Safety Data Sheet (SDS) Hydrogen Peroxide Solution, 30 - 32% W/W
Safety Data Sheet (SDS) Hydrogen Peroxide Solution, 30 - 32% w/w SECTION 1: Identification Product Identifier: BASELINE Hydrogen Peroxide Solution Master Product Number(s): S021001 EU Index Number: 008-003-00-9 Synonyms: Dihydrogen dioxide; Hydrogen dioxide; Hydroperoxide Chemical Names: DE Wasserstoffperoxid in Lösung; ES Peróxido de hidrógeno en disolución (Agua oxigenada); FR Peroxyde d'hydrogène en solution (Eau oxygénée); IT Perossido di idrogeno soluzione (Acqua ossigenata); NL Waterstofperoxide in oplossing Identified Uses: For laboratory use only. Not for drug, food, or household use. Manufacturer: SEASTAR CHEMICALS ULC 2061 Henry Avenue West, Sidney, BC V8L 5Z6 CANADA 1-250-655-5880 Emergency Number: CANUTEC (CAN): 1-613-996-6666 (24-hour) SECTION 2: Hazard identification GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) / WHMIS HPR / Regulation (EC) No 1272/2008 For the full text of the H-Statement(s) and P-Statement(s) mentioned in this Section, see Section 16. Classification: Serious eye damage – Category 1 Acute toxicity, Oral – Category 4 Acute toxicity, Inhalation – Category 4 GHS Label Elements Pictograms: Signal Word: Danger Hazard H318: Causes serious eye damage. Statements: H302 + H332: Harmful if swallowed or if inhaled. Precautionary P261: Avoid breathing fume/gas/mist/vapours/spray. Statements: P280: Wear protective gloves/protective clothing/eye protection/face protection. P301 + P312: IF SWALLOWED: Call a POISON CENTER or doctor if you feel unwell. P304 + P340: IF INHALED: Remove person to fresh air and keep comfortable for breathing. P305 + P351 + P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. -

Chlorotrifluoroethylene Interim AEGL Document
ACUTE EXPOSURE GUIDELINE LEVELS (AEGLs) FOR CHLOROTRIFLUOROETHYLENE (CAS Reg. No. 79-38-9) CF2 = CFCl INTERIM ACUTE EXPOSURE GUIDELINE LEVELS (AEGLs) FOR CHLOROTRIFLUOROETHYLENE (CAS Reg. No. 79-38-9) INTERIM CHLOROTRIFLUOROETHYLENE INTERIM: 06/2008/ Page 3 of 34 PREFACE Under the authority of the Federal Advisory Committee Act (FACA) P. L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review and interpret relevant toxicologic and other scientific data and develop AEGLs for high priority, acutely toxic chemicals. AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes to 8 hours. Three levels – AEGL-1, AEGL-2 and AEGL-3 C are developed for each of five exposure periods (10 and 30 minutes, 1 hour, 4 hours, and 8 hours) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows: AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, non-sensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure. AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape. -

Products Evolved During Hot Gas Welding of Fluoropolymers
Health and Safety Executive Products evolved during hot gas welding of fluoropolymers Prepared by the Health and Safety Laboratory for the Health and Safety Executive 2007 RR539 Research Report Health and Safety Executive Products evolved during hot gas welding of fluoropolymers Chris Keen BSc CertOH Mike Troughton BSc PhD CPhys MInstP Derrick Wake BSc, Ian Pengelly BSc, Emma Scobbie BSc Health and Safety Laboratory Broad Lane Sheffield S3 7HQ This report details the findings of a research project which was performed as a collaboration between the Health and Safety Executive (HSE) and The Welding Institute (TWI). The project aim was to identify and measure the amounts of products evolved during the hot gas welding of common fluoropolymers, to attempt to identify the causative agents of polymer fume fever. Carbonyl fluoride and/or hydrogen fluoride were detected from certain fluoropolymers when these materials were heated to their maximum welding temperatures. Significant amounts of ultrafine particles were detected from all of the fluoropolymers investigated when they were hot gas welded. The report concludes that fluoropolymers should be hot gas welded at the lowest possible temperature to reduce the potential for causing polymer fume fever in operators. If temperature control is not sufficient to prevent episodes of polymer fume fever, a good standard of local exhaust ventilation (LEV) should also be employed. This report and the work it describes were funded by the Health and Safety Executive (HSE). Its contents, including any opinions and/or conclusions expressed, are those of the authors alone and do not necessarily reflect HSE policy. HSE Books © Crown copyright 2007 First published 2007 All rights reserved. -

PVDF: a Fluoropolymer for Chemical Challenges
Electronically reprinted from August 2018 PVDF: A Fluoropolymer for Chemical Challenges When it comes to selecting materials of construction, keep in mind the favorable properties of fluoropolymers for corrosive service Averie Palovcak and Jason ince its commercialization in the Pomante, mid-1960s, polyvinylidene fluoride Arkema Inc. (PVDF) has been used across a Svariety of chemical process indus- tries (CPI) sectors due to its versatility and IN BRIEF broad attributes. With flagship applications PVDF AND THE in architectural coatings and the CPI, the FLUOROPOLYMER FAMILY breadth of industries where PVDF is utilized today is expansive. PVDF components (Fig- COPOLYMERS CHANGE ures 1 and 2) are utilized and installed where FLEXURAL PROPERTIES engineers are looking to maximize longevity PVDF COMPONENTS and reliability of process parts in many CPI sectors, including semiconductor, pharma- FIGURE 1. A variety of fluoropolymer components are shown ceutical, food and beverage, petrochemi- here cal, wire and cable, and general chemicals. change the performance properties. Fluo- PVDF and the fluoropolymer family ropolymers are divided into two main cat- PVDF is a high-performance plastic that falls egories: perfluorinated and partially fluori- into the family of materials called fluoropoly- nated [1]. The partially fluorinated polymers mers. Known for robust chemical resistance, contain hydrogen or other elements, while fluoropolymers are often utilized in areas the perfluorinated (fully fluorinated) poly- where high-temperature corrosion barriers mers are derivatives or copolymers of the are crucial. In addition to being chemically tetrafluoroethylene (C2F4) monomer. Com- resistant and non-rusting, this family of poly- monly used commercial fluoropolymers mers is also considered to have high purity, include polytetrafluoroethylene (PTFE), non-stick surfaces, good flame and smoke perfluoroalkoxy polymer (PFA), fluorinated resistance, excellent weathering and ultra- ethylene propylene (FEP), polyvinylidene violet (UV) stability. -

2004/12/27-LES Prefiled Exhibit 135-M, Hydrogen Fluoride Industry
I IJ Materials of Construction Guideline for Anhydrous Hydrogen Fluoride UPDATED: 12/27/04 LAST REVISION: January 2000 EXPIRATION DATE: 12/31/05 Dn1C.KETED USNRC February 24, 2006 (4:12pm) OFFICE OF SECRETARY RULEMAKINGS AND ADJUDICATIONS STAFF HYDROGEN FLUORIDE INDUSTRY PRACTICES INSTITUTE Docket No. 70-3103-ML Materials of Construction Task Group Materials of Construction Guideline for Anhydrous Hydrogen Fluoride The Hydrogen Fluoride Industry Practices Institute (HFIPI) is a subsidiary of the American Chemistry Council (ACC). LES Exhibit 135-M rem n/ate -se V- oea6 Materials of Construction Guideline for Anhydrous Hydrogen Fluoride UPDATED: 12/27/04 L. LAST REVISION: January 2000 EXPIRATION DATE: 12/31/05 MATERIALS OF CONSTRUCTION GUIDELINE FOR ANHYDROUS HYDROGEN FLUORIDE This document has been prepared by the "Materials of Construction Task Group" of the Hydrogen Fluoride Industry Practices Institute (HFIPI). The members of this Task Group participating in preparation of this guideline were: M. Howells (Chairman) Honeywell H. Jennings DuPont G. Navar* LCI/Norfluor E. Urban* AlliedSignal P.Wyatt Arkema Inc. William Heineken BWXT Y-12 Mike Berg Solvay Fluorides, LLC * Former Task Group member ©2004 Hydrogen Fluoride Industry Practices Institute This document cannot be copied or distributed without the express written consent of the Hydrogen Fluoride Industry Practices Institute ("HFIPI"). Copies of this document are available from the HFIPI, c/o Collier Shannon Scott, 3050 K Street, NW, Suite 400, Washington, DC 20007-5108. Materials -

ECTFE (HALAR®) AS a NEW MATERIAL for PRIMARY SAMPLE CONTAINMENT of ASTROMATERIALS. M. J. Calaway1 and J. T. Mcconnell2. 1
45th Lunar and Planetary Science Conference (2014) 1095.pdf ECTFE (HALAR®) AS A NEW MATERIAL FOR PRIMARY SAMPLE CONTAINMENT OF ASTROMATERIALS. M. J. Calaway1 and J. T. McConnell2. 1 Jacobs at NASA Johnson Space Center, Astromaterials Acquisition and Curation Office, Houston, TX, 77058; 2 NASA Intern at NASA Johnson Space Center, Houston, TX, Wyoming NASA Space Grant Consortium; [email protected]. Introduction: Fluoropolymers, such as Teflon® spectrometry (GC-MS), and Fourier transform (PTFE, PFA, FEP) and Viton® (FKM), have been infrared (FT-IR) spectroscopy. used for over 40 years in curating astromaterials at Particle Count Results: Liquid particle counts of NASA JSC. In general, fluoropolymers have low UPW during final rinse were taken with a HIAC outgassing and particle shedding properties that 3000A liquid syringe sampler with 8000A particle reduce cross-contamination to curated samples. counter. Figure 2 shows the results with Halar Ethylene – Chlorotrifluoroethylene (ECTFE), having very low particle shedding properties. commonly called Halar® (trademark of Solvay Particle HALAR (ECTFE) UPW Baseline Solexis), is a partially fluorinated semi-crystalline Diameter Particles Per 10 ml Particles Per 10 ml (μm) Average Average copolymer in the same class of fluoropolymers with 1 7.5 ± 4.7 5.0 ± 2.1 superior abrasion resistance and extremely low 3 4.8 ± 1.0 3.5 ± 1.1 permeability to liquids, gases, and vapors than any 5 4.0 ± 0.8 2.5 ± 0.4 10 3.5 ± 1.3 2.0 ± 0.7 other fluoropolymer (fig. 1). ECTFE coatings are 25 3.0 ± 0.8 1.0 ± 0.7 becoming more popular in the nuclear, 50 2.75 ± 1.0 1.0 ± 0.7 semiconductor, and biomedical industry for lining 100 2.75 ± 1.0 1.0 ± 0.7 150 2.75 ± 1.0 1.0 ± 0.7 isolation containment gloveboxes and critical piping Figure 2: Average measured particle counts of Halar. -

Chemical Resistance Guide
Pure Chemicals · Mixed Chemicals PVC · CPVC · PP · PVDF · PTFE · PFA EPDM · Viton® · Nitrile · CPE ISO 9001:2008 CERTIFIED chemical resistance guide CHEMICAL RESISTANCE GUIDE chemline.com Pure Chemicals · Mixed Chemicals PVC · CPVC · PP · PVDF · PTFE · PFA EPDM · Viton® · Nitrile · CPE ISO 9001:2008 CERTIFIED chemical resistance guide Chemical Resistance Guide page Materials of Construction 3-6 Pure Chemicals 7-33 Mixed Chemicals 33-34 2 | CHEMICAL RESISTANCE GUIDE CRG 6-12 | WWW.CHEMLINE.COM ©Chemline Plastics Limited 2014 Materials of Construction Note: Properties of plastics and elastomers vary because different PP (Pigmented Polypropylene) compounds of the same material are used for different products and Polypropylene (PP) is a thermoplastic polyolefin made from the olefin components. The following materials descriptions are of a general nature. propylene. A more modern term for polyolefin is polyalkene. Chemline Chemline should be consulted for material recommendations on specific offers piping systems, valves and controls normally in pigmented PP. The applications. addition of grey-beige pigment prevents degradation due to ultraviolet light penetration. THERMOPLASTICS PP is used in a wide variety of applications from acids and alkali’s to Most plastics are made from synthetic resins (polymers) through the organic solvents as well as pure water. PP is one of the best materials to process of polymerization. Two main types of plastics are thermoplastics use for systems exposed to varying pH levels, as many plastics do not and thermosets. Thermoplastic products are injection moulded or handle both acids and bases well. It is excellent on acids such hydrochloric extruded from compound material processed under heat and pressure. -

20210311 IAEG AD-DSL V5.0 for Pdf.Xlsx
IAEGTM AD-DSL Release Version 4.1 12-30-2020 Authority: IAEG Identity: AD-DSL Version number: 4.1 Issue Date: 2020-12-30 Key Yellow shading indicates AD-DSL family group entries, which can be expanded to display a non-exhaustive list of secondary CAS numbers belonging to the family group Substance Identification Change Log IAEG Regulatory Date First Parent Group IAEG ID CAS EC Name Synonyms Revision Date ECHA ID Entry Type Criteria Added IAEG ID IAEG000001 1327-53-3 215-481-4 Diarsenic trioxide Arsenic trioxide R1;R2;D1 2015-03-17 2015-03-17 100.014.075 Substance Direct Entry IAEG000002 1303-28-2 215-116-9 Diarsenic pentaoxide Arsenic pentoxide; Arsenic oxide R1;R2;D1 2015-03-17 2015-03-17 100.013.743 Substance Direct Entry IAEG000003 15606-95-8 427-700-2 Triethyl arsenate R1;R2;D1 2015-03-17 2017-08-14 100.102.611 Substance Direct Entry IAEG000004 7778-39-4 231-901-9 Arsenic acid R1;R2;D1 2015-03-17 2015-03-17 100.029.001 Substance Direct Entry IAEG000005 3687-31-8 222-979-5 Trilead diarsenate R1;R2;D1 2015-03-17 2017-08-14 100.020.890 Substance Direct Entry IAEG000006 7778-44-1 231-904-5 Calcium arsenate R1;R2;D1 2015-03-17 2017-08-14 100.029.003 Substance Direct Entry IAEG000009 12006-15-4 234-484-1 Cadmium arsenide Tricadmium diarsenide R1;R2;D1 2017-08-14 2017-08-14 Substance Direct Entry IAEG000021 7440-41-7 231-150-7 Beryllium (Be) R2 2015-03-17 2019-01-24 Substance Direct Entry IAEG000022 1306-19-0 215-146-2 Cadmium oxide R1;R2;D1 2015-03-17 2017-08-14 100.013.770 Substance Direct Entry IAEG000023 10108-64-2 233-296-7 Cadmium