Novel Methods Neue Verfahren Nouvelles Méthodes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2008/0317805 A1 Mckay Et Al
US 20080317805A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0317805 A1 McKay et al. (43) Pub. Date: Dec. 25, 2008 (54) LOCALLY ADMINISTRATED LOW DOSES Publication Classification OF CORTICOSTEROIDS (51) Int. Cl. A6II 3/566 (2006.01) (76) Inventors: William F. McKay, Memphis, TN A6II 3/56 (2006.01) (US); John Myers Zanella, A6IR 9/00 (2006.01) Cordova, TN (US); Christopher M. A6IP 25/04 (2006.01) Hobot, Tonka Bay, MN (US) (52) U.S. Cl. .......... 424/422:514/169; 514/179; 514/180 (57) ABSTRACT Correspondence Address: This invention provides for using a locally delivered low dose Medtronic Spinal and Biologics of a corticosteroid to treat pain caused by any inflammatory Attn: Noreen Johnson - IP Legal Department disease including sciatica, herniated disc, Stenosis, mylopa 2600 Sofamor Danek Drive thy, low back pain, facet pain, osteoarthritis, rheumatoid Memphis, TN38132 (US) arthritis, osteolysis, tendonitis, carpal tunnel syndrome, or tarsal tunnel syndrome. More specifically, a locally delivered low dose of a corticosteroid can be released into the epidural (21) Appl. No.: 11/765,040 space, perineural space, or the foramenal space at or near the site of a patient's pain by a drug pump or a biodegradable drug (22) Filed: Jun. 19, 2007 depot. E Day 7 8 Day 14 El Day 21 3OO 2OO OO OO Control Dexamethasone DexamethasOne Dexamethasone Fuocinolone Fluocinolone Fuocinolone 2.0 ng/hr 1Ong/hr 50 ng/hr 0.0032ng/hr 0.016 ng/hr 0.08 ng/hr Patent Application Publication Dec. 25, 2008 Sheet 1 of 2 US 2008/0317805 A1 900 ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 80.0 - 7OO – 6OO - 5OO - E Day 7 EDay 14 40.0 - : El Day 21 2OO - OO = OO – Dexamethasone Dexamethasone Dexamethasone Fuocinolone Fluocinolone Fuocinolone 2.0 ng/hr 1Ong/hr 50 ng/hr O.OO32ng/hr O.016 ng/hr 0.08 nghr Patent Application Publication Dec. -

Nitrate Prodrugs Able to Release Nitric Oxide in a Controlled and Selective
Europäisches Patentamt *EP001336602A1* (19) European Patent Office Office européen des brevets (11) EP 1 336 602 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.7: C07C 205/00, A61K 31/00 20.08.2003 Bulletin 2003/34 (21) Application number: 02425075.5 (22) Date of filing: 13.02.2002 (84) Designated Contracting States: (71) Applicant: Scaramuzzino, Giovanni AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU 20052 Monza (Milano) (IT) MC NL PT SE TR Designated Extension States: (72) Inventor: Scaramuzzino, Giovanni AL LT LV MK RO SI 20052 Monza (Milano) (IT) (54) Nitrate prodrugs able to release nitric oxide in a controlled and selective way and their use for prevention and treatment of inflammatory, ischemic and proliferative diseases (57) New pharmaceutical compounds of general effects and for this reason they are useful for the prep- formula (I): F-(X)q where q is an integer from 1 to 5, pref- aration of medicines for prevention and treatment of in- erably 1; -F is chosen among drugs described in the text, flammatory, ischemic, degenerative and proliferative -X is chosen among 4 groups -M, -T, -V and -Y as de- diseases of musculoskeletal, tegumental, respiratory, scribed in the text. gastrointestinal, genito-urinary and central nervous sys- The compounds of general formula (I) are nitrate tems. prodrugs which can release nitric oxide in vivo in a con- trolled and selective way and without hypotensive side EP 1 336 602 A1 Printed by Jouve, 75001 PARIS (FR) EP 1 336 602 A1 Description [0001] The present invention relates to new nitrate prodrugs which can release nitric oxide in vivo in a controlled and selective way and without the side effects typical of nitrate vasodilators drugs. -

18 December 2020 – to Date)
(18 December 2020 – to date) MEDICINES AND RELATED SUBSTANCES ACT 101 OF 1965 (Gazette No. 1171, Notice No. 1002 dated 7 July 1965. Commencement date: 1 April 1966 [Proc. No. 94, Gazette No. 1413] SCHEDULES Government Notice 935 in Government Gazette 31387 dated 5 September 2008. Commencement date: 5 September 2008. As amended by: Government Notice R1230 in Government Gazette 32838 dated 31 December 2009. Commencement date: 31 December 2009. Government Notice R227 in Government Gazette 35149 dated 15 March 2012. Commencement date: 15 March 2012. Government Notice R674 in Government Gazette 36827 dated 13 September 2013. Commencement date: 13 September 2013. Government Notice R690 in Government Gazette 36850 dated 20 September 2013. Commencement date: 20 September 2013. Government Notice R104 in Government Gazette 37318 dated 11 February 2014. Commencement date: 11 February 2014. Government Notice R352 in Government Gazette 37622 dated 8 May 2014. Commencement date: 8 May 2014. Government Notice R234 in Government Gazette 38586 dated 20 March 2015. Commencement date: 20 March 2015. Government Notice 254 in Government Gazette 39815 dated 15 March 2016. Commencement date: 15 March 2016. Government Notice 620 in Government Gazette 40041 dated 3 June 2016. Commencement date: 3 June 2016. Prepared by: Page 2 of 199 Government Notice 748 in Government Gazette 41009 dated 28 July 2017. Commencement date: 28 July 2017. Government Notice 1261 in Government Gazette 41256 dated 17 November 2017. Commencement date: 17 November 2017. Government Notice R1098 in Government Gazette 41971 dated 12 October 2018. Commencement date: 12 October 2018. Government Notice R1262 in Government Gazette 42052 dated 23 November 2018. -

Supplementary Information
Supplementary Information Network-based Drug Repurposing for Novel Coronavirus 2019-nCoV Yadi Zhou1,#, Yuan Hou1,#, Jiayu Shen1, Yin Huang1, William Martin1, Feixiong Cheng1-3,* 1Genomic Medicine Institute, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195, USA 2Department of Molecular Medicine, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, OH 44195, USA 3Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH 44106, USA #Equal contribution *Correspondence to: Feixiong Cheng, PhD Lerner Research Institute Cleveland Clinic Tel: +1-216-444-7654; Fax: +1-216-636-0009 Email: [email protected] Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. Supplementary Table S2. Protein sequence identities across 5 protein regions in 15 coronaviruses. Supplementary Table S3. HCoV-associated host proteins with references. Supplementary Table S4. Repurposable drugs predicted by network-based approaches. Supplementary Table S5. Network proximity results for 2,938 drugs against pan-human coronavirus (CoV) and individual CoVs. Supplementary Table S6. Network-predicted drug combinations for all the drug pairs from the top 16 high-confidence repurposable drugs. 1 Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. GenBank ID Coronavirus Identity % Host Location discovered MN908947 2019-nCoV[Wuhan-Hu-1] 100 Human China MN938384 2019-nCoV[HKU-SZ-002a] 99.99 Human China MN975262 -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Lung Inflammation and Atopy Muscarinic Receptor Expression
The Journal of Immunology Maternal Exposure to Secondhand Cigarette Smoke Primes the Lung for Induction of Phosphodiesterase-4D5 Isozyme and Exacerbated Th2 Responses: Rolipram Attenuates the Airway Hyperreactivity and Muscarinic Receptor Expression but Not Lung Inflammation and Atopy1 Shashi P. Singh,* Neerad C. Mishra,* Jules Rir-sima-ah,* Mathew Campen,* Viswanath Kurup,† Seddigheh Razani-Boroujerdi,* and Mohan L. Sopori2* Airway hyperreactivity (AHR), lung inflammation, and atopy are clinical signs of allergic asthma. Gestational exposure to ciga- rette smoke (CS) markedly increases the risk for childhood allergic asthma. Muscarinic receptors regulate airway smooth muscle tone, and asthmatics exhibit increased AHR to muscarinic agonists. We have previously reported that in a murine model of bronchopulmonary aspergillosis, maternal exposure to mainstream CS increases AHR after acute intratracheal administration of Aspergillus fumigatus extract. However, the mechanism by which gestational CS induces allergic asthma is unclear. We now show for the first time that, compared with controls, mice exposed prenatally to secondhand CS exhibit increased lung inflammation (predominant infiltration by eosinophils and polymorphs), atopy, and airway resistance, and produce proinflammatory cytokines (IL-4, IL-5, IL-6, and IL-13, but not IL-2 or IFN-␥). These changes, which occur only after an allergen (A. fumigatus extract) treatment, are correlated with marked up-regulated lung expression of M1, M2, and M3 muscarinic receptors and phosphodi- esterase (PDE)4D5 isozyme. Interestingly, the PDE4-selective inhibitor rolipram attenuates the increase in AHR, muscarinic receptors, and PDE4D5, but fails to down-regulate lung inflammation, Th2 cytokines, or serum IgE levels. Thus, the fetus is extraordinarily sensitive to CS, inducing allergic asthma after postnatal exposure to allergens. -

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances
WHO/PSM/QSM/2006.3 The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances 2006 Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines Medicines Policy and Standards The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Hexoprenaline: Β-Adrenoreceptor Selectivity in Isolated Tissues from the Guinea-Pig
Clinical and Experimental Pharmacology and Physiology (1975) 2, 541-547. Hexoprenaline: p-adrenoreceptor selectivity in isolated tissues from the guinea-pig Stella R. O’Donnell and Janet C. Wanstall Department of Physiology, University of Queenstand, Brisbane, Australia (Received 12 March 1975; revision received 22 April 1975) SUMMARY 1. A catecholamine P-adrenoreceptor agonist, hexoprenaline, was examined in vitro on five guinea-pig tissues and its potency relative to isoprenaline (as 100) obtained. 2. Hexoprenaline clearly delineated between those tissues classified as containing P,-adrenoreceptors (trachea, hind limb blood vessels and uterus; relative potencies 219, 110 and 76 respectively) and those classified as containing P,-adrenoreceptors (atria and ileum; relative potencies 3.3 and 1.0 respectively). 3. Hexoprenaline differed from some previously studied noncatecholamine P-adrenoreceptor agonists in being only two-fold less potent, relative to isoprena- line, as a vasodilator in perfused hind limb than as a tracheal relaxant. Key words : j3-adrenoreceptors, blood vessels, bronchodilators, guinea-pig, hexo- prenaline, selectivity, trachea. INTRODUCTION In a previous study using in vitro preparations from the guinea-pig, O’Donnell & Wanstall (1974a) examined potential sympathomimetic bronchodilator compounds for their potency as tracheal relaxants (p,-adrenoreceptors), atrial stimulants (P,-adrenoreceptors) and vasodilators in the perfused hind limb (p2-adrenoreceptors). Some resorcinolamines showed selectivity for trachea compared with not only atria but also blood vessels. There is some evidence in dogs in vivo that other noncatecholamines, namely carbuterol (Wardell et al., 1974), and salbutamol and terbutaline (Wasserman & Levy, 1974), display similar select- ivity. If selectivity between respiratory and vascular smooth muscle represents selectivity at the p-adrenoreceptor level, it poses the question whether the P1/p2subclassification (Lands et al., 1967a; Lands, Luduena & Buzzo, 1967b) is too rigid. -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -

(12) Patent Application Publication (10) Pub. No.: US 2009/0099225A1 Freund Et Al
US 20090099225A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0099225A1 Freund et al. (43) Pub. Date: Apr. 16, 2009 (54) METHOD FOR THE PRODUCTION OF Related U.S. Application Data PROPELLANT GAS-FREE AEROSOLS FROM (63) Continuation of application No. 1 1/506,128, filed on AQUEOUSMEDICAMENT PREPARATIONS Aug. 17, 2006, now Pat. No. 7,470,422, which is a continuation of application No. 10/417.766, filed on (75) Inventors: Bernhard Freund, Gau-Algesheim Apr. 17, 2003, now abandoned, which is a continuation (DE); Bernd Zierenberg, Bingen of application No. 09/331,023, filed on Sep. 15, 1999, am Rhein (DE) now abandoned. (30) Foreign Application Priority Data Correspondence Address: MICHAEL P. MORRIS Dec. 20, 1996 (DE) ............................... 19653969.2 BOEHRINGERINGELHEMI USA CORPORA Dec. 16, 1997 (EP) ......................... PCT/EP97/07062 TION Publication Classification 900 RIDGEBURY RD, P. O. BOX 368 RIDGEFIELD, CT 06877-0368 (US) (51) Int. Cl. A63L/46 (2006.01) (73) Assignee: Boehringer Ingelheim Pharma A63L/437 (2006.01) KG, Ingelheim (DE) (52) U.S. Cl. ......................................... 514/291; 514/299 (57) ABSTRACT (21) Appl. No.: 12/338,812 The present invention relates to pharmaceutical preparations in the form of aqueous solutions for the production of propel (22) Filed: Dec. 18, 2008 lant-free aerosols. US 2009/0099225A1 Apr. 16, 2009 METHOD FOR THE PRODUCTION OF 0008 All substances which are suitable for application by PROPELLANT GAS-FREE AEROSOLS FROM inhalation and which are soluble in the specified solvent can AQUEOUSMEDICAMENT PREPARATIONS be used as pharmaceuticals in the new preparations. Pharma ceuticals for the treatment of diseases of the respiratory pas RELATED APPLICATIONS sages are of especial interest. -
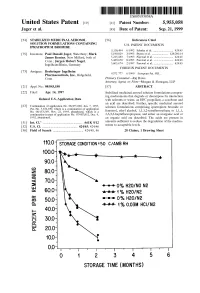
100 Storage Condition=50 C/Ambrh
USOO595.5058A United States Patent (19) 11 Patent Number: S.9SS,0589 9 Jager et al. (45) Date of Patent: Sep. 21,9 1999 54). STABILIZED MEDICINAL AEROSOL 56) References Cited SOLUTION FORMULATIONS CONTAINING U.S. PATENT DOCUMENTS IPRATROPIUM BROMIDE a 5,118,494 6/1992 Schultz et al. ............................ 424/45 75 Inventors: Paul Donald Jager, Waterbury; Mark 5,190,029 3/1993 Byron et al. ... ... 128/200.14 James Kontny, New Milford, both of 5,225,183 7/1993 Purewal et al. ........................... 424/45 Conn.; Jurgen Hubert Nagel 5.439,670 8/1995 Purewal et al. ... 424/45 Ingelheim/Rhein, Germany s 5,605,674 2/1997 Purewal et al. ........................... 424/45 FOREIGN PATENT DOCUMENTS 73 Assignee: Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, 0372 777 6/1990 European Pat. Off.. Conn. Primary Examiner Raj Bawa Attorney, Agent, or Firm Morgan & Finnegan, LLP 21 Appl.pp No.: 08/843,180 57 ABSTRACT 22 Filed: Apr. 14, 1997 Stabilized medicinal aeroSol Solution formulations compris O O ing medicaments that degrade or decompose by interaction Related U.S. Application Data with solvents or water, an HFC propellant, a cosolvent and an acid are described. Further, Specific medicinal aeroSol 63 Staggypt.NE "A iGs Solution formulations comprising ipratropium bromide or No. 08/153.549, Nov. 22, 1993, abandoned E. is a fenoterol, ethyl alcohol, 1,1,1,2-tetrafluoroethane or 1,1,1, continuation-in-part of application No. 07/987,852, Dec. 9, 2,3,3,3-heptafluoropropane, and either an inorganic acid or 1992, abandoned. an organic acid are described. The acids are present in 51 Int.