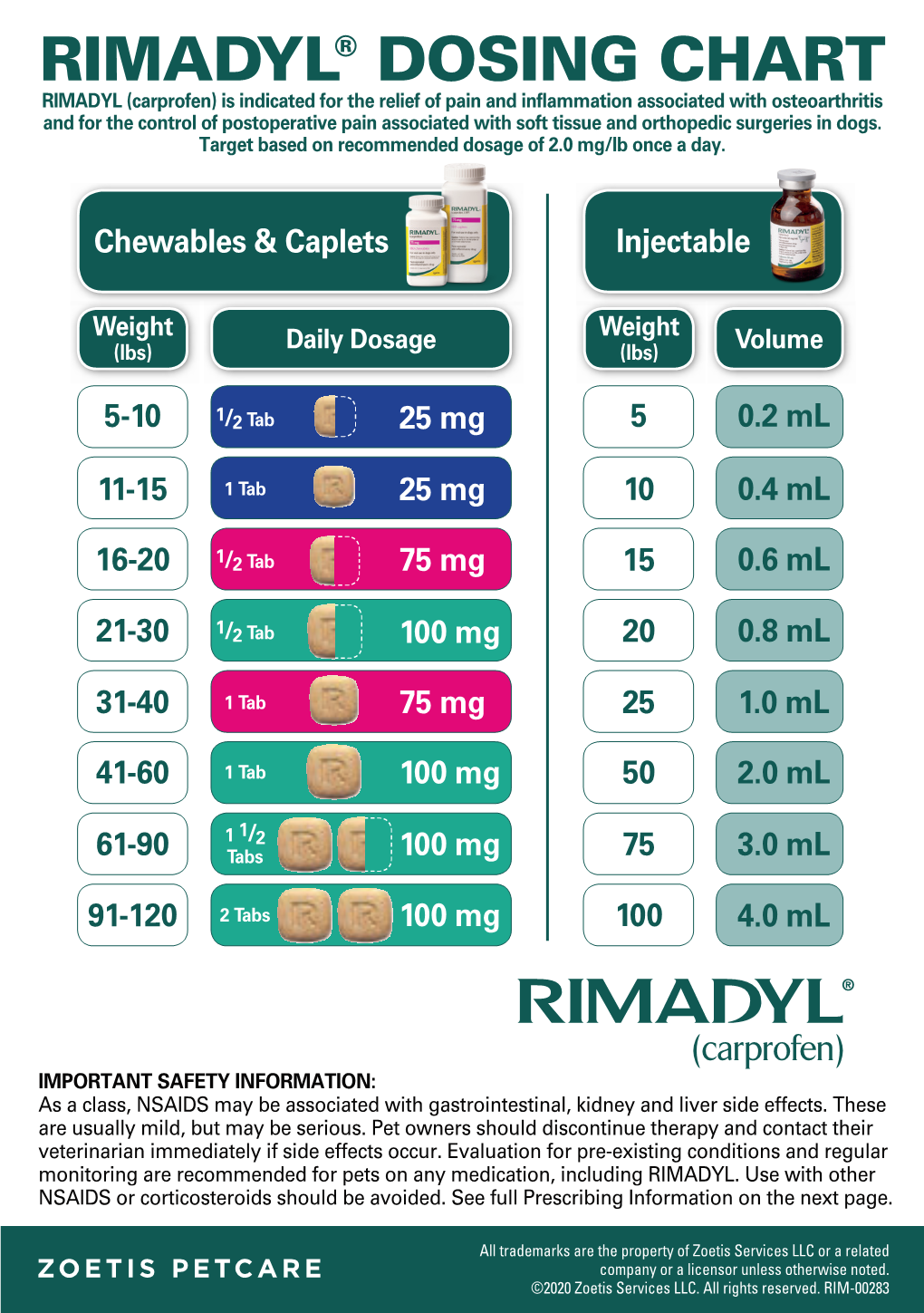
Rimadyl® Dosing Chart
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent (19) 11 Patent Number: 5,955,504 Wechter Et Al
USOO5955504A United States Patent (19) 11 Patent Number: 5,955,504 Wechter et al. (45) Date of Patent: Sep. 21, 1999 54 COLORECTAL CHEMOPROTECTIVE Marnett, “Aspirin and the Potential Role of Prostaglandins COMPOSITION AND METHOD OF in Colon Cancer, Cancer Research, 1992; 52:5575–89. PREVENTING COLORECTAL CANCER Welberg et al., “Proliferation Rate of Colonic Mucosa in Normal Subjects and Patients with Colonic Neoplasms: A 75 Inventors: William J. Wechter; John D. Refined Immunohistochemical Method.” J. Clin Pathol, McCracken, both of Redlands, Calif. 1990; 43:453-456. Thun et al., “Aspirin Use and Reduced Risk of Fatal Colon 73 Assignee: Loma Linda University Medical Cancer." N Engl J Med 1991; 325:1593-6. Center, Loma Linda, Calif. Peleg, et al., “Aspirin and Nonsteroidal Anti-inflammatory Drug Use and the Risk of Subsequent Colorectal Cancer.” 21 Appl. No.: 08/402,797 Arch Intern Med. 1994, 154:394–399. 22 Filed: Mar 13, 1995 Gridley, et al., “Incidence of Cancer among Patients With Rheumatoid Arthritis J. Natl Cancer Inst 1993 85:307-311. 51) Int. Cl. .......................... A61K 31/19; A61K 31/40; Labayle, et al., “Sulindac Causes Regression Of Rectal A61K 31/42 Polyps. In Familial Adenomatous Polyposis” Gastroenterol 52 U.S. Cl. .......................... 514/568; 514/569; 514/428; ogy 1991 101:635-639. 514/416; 514/375 Rigau, et al., “Effects Of Long-Term Sulindac Therapy On 58 Field of Search ..................................... 514/568, 570, Colonic Polyposis” Annals of Internal Medicine 1991 514/569, 428, 416, 375 11.5:952-954. Giardiello.et al., “Treatment Of Colonic and Rectal 56) References Cited Adenomas With Sulindac In Familial Adenomatous Poly U.S. -

NIH Public Access Author Manuscript J Am Chem Soc
NIH Public Access Author Manuscript J Am Chem Soc. Author manuscript; available in PMC 2014 January 09. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: J Am Chem Soc. 2013 January 9; 135(1): 22–25. doi:10.1021/ja308733u. A binding site for non-steroidal anti-inflammatory drugs in FAAH Laura Bertolacci¶, Elisa Romeo¶, Marina Veronesi, Paola Magotti, Clara Albani, Mauro Dionisi, Chiara Lambruschini, Rita Scarpelli, Andrea Cavalli‡, Marco De Vivo, Daniele Piomelli*,†, and Gianpiero Garau* ‡Department of Pharmaceutical. Sciences, University of Bologna, Italy. †Department of Pharmacology, Univ. of California, Irvine, California, USA. Abstract In addition to inhibiting the cyclooxygenasemediated biosynthesis of prostanoids, various widely used non-steroidal anti-inflammatory drugs (NSAIDs) enhance endocannabinoid signaling by blocking the anandamidedegrading membrane enzyme, fatty acid amide hydrolase (FAAH). The X-ray structure of FAAH in complex with the NSAID carprofen, along with studies of site- directed mutagenesis, enzyme activity assays, and nuclear magnetic resonance, now reveal the molecular details of this interaction, providing information that may guide the design of dual FAAH-cyclooxygenase inhibitors with superior analgesic efficacy. Non-steroidal anti-inflammatory drugs (NSAIDs), one of the most widely used classes of therapeutic agents, alleviate pain and inflammation1 by inhibiting the enzymes cyclooxygenase-1 (COX-1) and COX-2,2 which catalyze the conversion of -

(12) United States Patent (10) Patent No.: US 6,472,433 B2 Wechter (45) Date of Patent: Oct
USOO6472433B2 (12) United States Patent (10) Patent No.: US 6,472,433 B2 Wechter (45) Date of Patent: Oct. 29, 2002 (54) METHOD FOR TREATMENT OF Variability of Inversion of (R)-Flurbiprofen in Different NFLAMMATION WITH R-NSAIDS Species, Sabine Menzel-Soglowek, Gerd Geisslinger, Win fried S. Beck, and Kay Brune-Journal of Pharmaceutical (75) Inventor: William J. Wechter, Ojai, CA (US) Sciences vol. 81, No. 9, Sep. 1992. Disposition and Pharmacokinetics of R-flurbiprofen in (73) Assignee: Loma Linda University Medical Three Species: Demonstration of R- to S-Flurbiprofen Center, Loma Linda, CA (US) Inversion in the Mouse, Rat and Monkey-William J. Wechter, E. David Murray, Jr. Karina M. Gibson, David D. (*) Notice: Subject to any disclaimer, the term of this Quiggle, and Douglas L. Leipold-Laboratory of Chemical patent is extended or adjusted under 35 Endocrinology, Loma Linda University School of Medicine, U.S.C. 154(b) by 0 days. 1998. Superaspirin, Jerome Groopman The New Yorker, Jun. 15, (21) Appl. No.: 09/797,022 1998 pp. 32–35. (22) Filed: Mar. 1, 2001 Building a Better Aspirin, Science, vol. 280, May 22, 1998. (65) Prior Publication Data R-Flurbiprofen Chemoprevention and Treatment of Intesti nal Adenomas in the APC Min/+ Mouse Model: Implica US 2001/0012849 A1 Aug. 9, 2001 tions for Prophylazis and Treatment of Colon Caner, Will iam Wechter, Darko Kantoci, E. David Murray, Jr. David D. Related U.S. Application Data Quiggle, Douglas D. Leipold, Karina M. Gibson, and John D. McCracker-Cancer Research 57, 4316-4324, Oct. 1, (63) Continuation of application No. -

Rimadyl (Carprofen)
Carprofen (kar - pro -fen ) Category: Non -Steroidal Antiinflammatory Drug (NSAID) Other Names for this Medication:Rimadyl®, Carprieve®, Norocarp®, Novox®, quellin®, Vetprofen®. Common Dosage Forms: Veterinary: 25 mg, 75 mg, & 100 mg scored caplets (tablets) and chewable tablets. Human: None. This information sheet does not contain all available information for this medication. It is to help answer commonly asked questions and help you give the medication safely and effectively to your animal. If you have other questions or need more information about this medication, contact your veterinarian or pharmacist. well it worked or didn’t work. Key Information Y If your pet is pregnant or nursing, talk to your veterinarian Y Can give with or without food; food may reduce the about the risks of using this drug. chances for stomach problems. Y Tell your veterinarian and pharmacist about any medication Y Most dogs usually tolerate this drug well, but some will side effects (including allergic reactions, lack of appetite, (rarely) develop stomach ulcers or serious kidney and liver diarrhea, itching, hair loss) your pet has developed in the problems. Watch for: Increased or decreased appetite (eating more or less than normal); vomiting; changes in past. bowel movements; changes in behavior or activity level When should this medication not be used or be used (more or less active than normal); incoordination or weakness (eg, stumbling, clumsiness); seizures very carefully? (convulsions); aggression (threatening behavior/actions); No drug is 100% safe in all patients, but your veterinarian will yellowing of gums, skin, or whites of the eyes (jaundice); discuss with you any specific concerns about using this drug in changes in drinking habits (frequency, amount consumed) your pet. -

Unthsc Unthsc
Institutional Animal Care and Use Committee Title: Analgesics and Anesthesia in Laboratory Animals at UNTHSC UNTHSC Document #: 035 Version #: 02 Approved by IACUC Date: August 22, 2017 A. BACKGROUND INFORMATION a. In general, procedures which cause pain in humans should be expected to cause pain in animals. b. Appropriate analgesics must be used unless withholding such agents is scientifically justified in the animal use protocol. B. RESPONSIBILITIES a. It is the responsibility of the Principal Investigator (PI): i. To list appropriate analgesics when performing potentially painful procedures on animals. The PI must consult with the Attending Veterinarian for information on which analgesic(s) to use if the PI is unsure. ii. To procure the analgesics listed on an approved protocol unless arrangements are made with DLAM (Department of Laboratory Animal Medicine) ahead of time. Some analgesics are controlled substances and will require a DEA license. It is the responsibility of the PI to have this license. b. It is the responsibility of the Principal Investigator and other research personnel who will administer analgesics to have completed the applicable CITI training module. c. It is the responsibility of the Principal Investigator or designated lab staff and/or students to administer the analgesics listed in the approved protocol unless arrangements are made ahead of time for DLAM staff to do so. d. It is the responsibility of IACUC to assure that this SOP is followed. C. PROCEDURES a. Determining which procedures require analgesia and which ones may be useful, several factors should be considered: i. The invasiveness of the procedure that was performed: 1. -

Nsaids in Cats
ISFM and AAFP Consensus Guidelines Long-term use of NSAIDs in cats Clinical Practice A preprint from the Journal of Feline Medicine and Surgery Volume 12, July 2010 Journal of Feline Medicine and Surgery (2010) 12, 519 doi:10.1016/j.jfms.2010.05.003 EDITORIAL NSAIDs and cats – it’s been a long journey Although the first This issue of JFMS contains the first ever panel have covered much valuable ground: international consensus guidelines ✜ To set the scene they consider how use of NSAIDs on the long-term use of non-steroidal common chronic pain can be in cats, typically was probably anti-inflammatory drugs (NSAIDs) in cats. related to degenerative joint disease, This timely publication, which appears on idiopathic cystitis, trauma and cancer. by Hippocrates, pages 521–538 (doi:10.1016/j.jfms.2010.05.004), ✜ They then explain how and why NSAIDs is a collaborative enterprise by the International can have such positive and, potentially, it has taken until Society of Feline Medicine (ISFM) and negative actions. now for cats to American Association of Feline Practitioners ✜ They consider the best ways of enhancing (AAFP). It has been compiled by a panel of owner and cat compliance, make suggestions gain the benefit of world leaders in the understanding of pain about sensible dosing frequencies, timing of in cats and, without doubt, is essential reading medication and accuracy of dosing, and the long-term use for all small animal veterinary surgeons. emphasize the importance of always using of these drugs. It is interesting to reflect that although the ‘lowest effective dose’. -

Pharmacokinetics and Bioavailability of Carprofen in Rainbow Trout (Oncorhynchus Mykiss) Broodstock
pharmaceutics Article Pharmacokinetics and Bioavailability of Carprofen in Rainbow Trout (Oncorhynchus mykiss) Broodstock Kamil Uney 1,*, Duygu Durna Corum 2 , Ertugrul Terzi 3 and Orhan Corum 2 1 Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, University of Selcuk, Konya 42130, Turkey 2 Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, University of Kastamonu, Kastamonu 37150, Turkey; [email protected] (D.D.C.); [email protected] (O.C.) 3 Faculty of Fisheries, University of Kastamonu, Kastamonu 37150, Turkey; [email protected] * Correspondence: [email protected]; Tel.: +90-332-223-2733 Abstract: The aim of this study was to determine the pharmacokinetics of carprofen following intra- venous (IV), intramuscular (IM) and oral routes to rainbow trout (Oncorhynchus mykiss) broodstock at temperatures of 10 ± 1.5 ◦C. In this study, thirty-six healthy rainbow trout broodstock (body weight, 1.45 ± 0.30 kg) were used. The plasma concentrations of carprofen were determined using high-performance liquid chromatography and pharmacokinetic parameters were calculated using non-compartmental analysis. Carprofen was measured up to 192 h for IV route and 240 h for IM, and oral routes in plasma. The elimination half-life (t λ ) was 30.66, 46.11, and 41.08 h for IV, IM and 1/2 z oral routes, respectively. Carprofen for the IV route showed the total clearance of 0.02 L/h/kg and volume of distribution at steady state of 0.60 L/kg. For IM and oral routes, the peak plasma concen- Citation: Uney, K.; Durna Corum, D.; tration (C ) was 3.96 and 2.52 µg/mL with the time to reach C of 2 and 4 h, respectively. -

DNA Replication Is the Target for the Antibacterial Effects of Nonsteroidal Anti-Inflammatory Drugs
University of Wollongong Research Online Faculty of Science, Medicine and Health - Papers: part A Faculty of Science, Medicine and Health 1-1-2014 DNA replication is the target for the antibacterial effects of nonsteroidal anti-inflammatory drugs Zhou Yin University of Wollongong, [email protected] Yao Wang University of Wollongong, [email protected] Louise Whittell University of Wollongong, [email protected] Slobodan Jergic University of Wollongong, [email protected] Michael Liu University of Technology Sydney See next page for additional authors Follow this and additional works at: https://ro.uow.edu.au/smhpapers Part of the Medicine and Health Sciences Commons, and the Social and Behavioral Sciences Commons Recommended Citation Yin, Zhou; Wang, Yao; Whittell, Louise; Jergic, Slobodan; Liu, Michael; Harry, Elizabeth J.; Dixon, Nicholas; Kelso, Michael; Beck, Jennifer; and Oakley, Aaron, "DNA replication is the target for the antibacterial effects of nonsteroidal anti-inflammatory drugs" (2014). Faculty of Science, Medicine and Health - Papers: part A. 1685. https://ro.uow.edu.au/smhpapers/1685 Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] DNA replication is the target for the antibacterial effects of nonsteroidal anti- inflammatory drugs Abstract Evidence suggests that some nonsteroidal anti-inflammatory drugs (NSAIDs) possess antibacterial properties with an unknown mechanism. We describe the in vitro antibacterial properties of the NSAIDs carprofen, bromfenac, and vedaprofen, and show that these NSAIDs inhibit the Escherichia coli DNA polymerase III β subunit, an essential interaction hub that acts as a mobile tether on DNA for many essential partner proteins in DNA replication and repair. -

Guidelines for the Assessment and Management of Pain in Rodents and Rabbits
Guidelines for the Assessment and Management of Pain in Rodents and Rabbits Preface The assessment and management of pain in humans and animals has generated unprecedented discussion in the last 20 years. In the veterinary field, heightened awareness to pain has prompted several professional organizations to make formal position statements, which unequivocally mandate pain relief (McMillan, 2003). Regulatory bodies primarily concerned with the use of animals in biomedical research and teaching have also had an increased interest in pain and on July 10, 2003 the USDA's Animal and Plant Health Inspection Service (APHIS) published a Federal Register notice calling for comments on the definition and reporting of pain and distress in animals under the Animal Welfare Act. While there is much discussion on the continuum of stress, distress and pain in animals, there is little agreement on the difficulty of delineating crossover points for these apparently overlapping sensations. In addition, there is no agreed objective measure, which can be used to reliably identify pain in animals and its severity. Further and more difficult, is the assessment of different types of pain. For example, while there is an intuitive predilection to consider “real” pain as physical pain, several studies suggest psychological distress can also adversely affect animal welfare (McMillan, 2003). Semantics aside, it is generally agreed that pain adversely impacts the welfare of animals and that in research protocols, pain, if not controlled, is a variable, which can confound the interpretation of experimental results. A position statement of the American College of Laboratory Animal Medicine (ACLAM) declares that “Procedures expected to cause more than slight or momentary pain (e.g., pain in excess of a needle prick or injection) require the appropriate use of pain-relieving measures unless scientifically justified in an approved animal care and use protocol.”(http://www.aclam.org/pub_pain_distress.html). -

Carprofen: a Vulture-Toxic Drug
Carprofen: a vulture-toxic drug Summary of evidence that carprofen is toxic to vultures; and that meloxicam, an alternative to carprofen, is not toxic to vultures as well as a safe and effective veterinary drug Version 1: January 2021 SAVE is an international consortium of conservation and research organisations whose mission is to respond to the vulture crisis in Asia by striving to halt vulture population declines and working to minimise their negative impacts on ecological and human health. For further details go to www.save-vultures.org Carprofen: a vulture-toxic drug SAVE 01.2021 Carprofen is toxic to Gyps vultures; however, meloxicam is not toxic to Gyps vultures and an effective alternative to carprofen for veterinary purposes Aim This paper, intended for decision-makers involved in drug licensing, presents evidence of the toxicity of carprofen, a non-steroidal anti-inflammatory drug (NSAID), to Gyps vultures; and the safety, to both vultures and domesticated animals, and effectiveness, in domesticated animals, of meloxicam, an alternative NSAID. Executive summary 1. The NSAID carprofen can be toxic to Gyps vultures at high, but realistic, concentrations. 2. Captive vultures are exposed to NSAIDs through veterinary care. 3. A survey of wildlife veterinarians found that treatment with carprofen resulted in visceral gout and death in 1 out of 17 cases involving a Gyps vulture. 4. The maximum level of exposure (MLE) of a given drug to a Gyps vulture is intended to reflect the largest dose of the drug likely to be encountered by that species in the wild. The MLE for an NSAID is calculated from the body weight of a given vulture species, the weight of a large meal for that species and highest tissue-specific residue concentration of the NSAID in tissues of a domesticated ungulate treated with the drug. -
Carprofen Caplets DOG OWNER INFORMA TION
-DO NOT REMOVE- -DO NOT Carprofen Caplets INFORMATION DOG OWNER Dog Owner Information about Carprofen Caplets for Osteoarthritis and Post-Surgical Pain This summary contains important information about Carprofen What are the possible side effects that may occur in my dog Caplets. You should read this information before you start giving your during Carprofen Caplets therapy? dog Carprofen Caplets and review it each time the prescription is Carprofen Caplets, like other drugs, may cause some side effects. refilled. This sheet is provided only as a summary and does not take Serious but rare side effects have been reported in dogs taking NSAIDs, the place of instructions from your veterinarian. Talk to your including carprofen. Serious side effects can occur with or without veterinarian if you do not understand any of this information or if you warning and in rare situations result in death. want to know more about Carprofen Caplets. The most common NSAID-related side effects generally involve the What are Carprofen Caplets? stomach (such as bleeding ulcers), and liver or kidney problems. Look Carprofen Caplets are a nonsteroidal anti-inflammatory drug (NSAID) for the following side effects that can indicate your dog may be having that is used to reduce pain and inflammation (soreness) due to a problem with Carprofen Caplets or may have another medical osteoarthritis and pain following surgery in dogs. Carprofen Caplets problem: are a prescription drug for dogs. It is available as a caplet and is given • Decrease or increase in appetite to -
Non-Steroidal Anti-Inflammatory Drugs in Food Producing Animals
American Journal of Animal and Veterinary Sciences Review Articles Non-Steroidal Anti-Inflammatory Drugs in Food Producing Animals De Vito Virginia Department of Veterinary Medicine, University of Sassari, Via Vienna 2, 07100 Sassari, Italy Article history Abstract: Providing adequate food supply to meet the demands of a Received: 29-12-2014 growing population is a challenge for this century. The food producing Revised: 14-01-2015 animals bred in optimized conditions supply a large amount of food Accepted: 04-02-2015 derivatives. At the same time, society has become progressively more Email: [email protected] concerned about animal suffering and aware of the need of pain prevention and treatments. One of the measures aimed at improving animals welfare is pain management a rational and controlled use of the anti-inflammatory drugs such as Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). However, NSAIDs residues in biological matrices are still an issue. In addition veterinary pharmacology still has a reduced drug armamentarium if compared with human medicine. In order to address these shortfalls investigations on new NSAIDs (off label drug) in food producing animals are needed. Keywords: Pain Management, Food Producing Animals, Non-Steroidal Anti-Inflammatory Drugs, Off Label Drugs, Residues Introduction reactions that often induce negative effects on well-being and behaviour, as well as on growth and reproduction of Over the last decades, animal production science the animals. However, the main obstacle for the pain supported the modernization of food animal husbandry in management in farm animals is to recognise, quantify order to provide adequate food supply to meet the demands and evaluate the pain status of each individual in a farm of a growing population.