Ergot Alkaloids Contribute to Virulence in an Insect Model of Invasive Aspergillosis Received: 1 June 2017 Daniel G
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Ergot Alkaloids Mycotoxins in Cereals and Cereal-Derived Food Products: Characteristics, Toxicity, Prevalence, and Control Strategies
agronomy Review Ergot Alkaloids Mycotoxins in Cereals and Cereal-Derived Food Products: Characteristics, Toxicity, Prevalence, and Control Strategies Sofia Agriopoulou Department of Food Science and Technology, University of the Peloponnese, Antikalamos, 24100 Kalamata, Greece; [email protected]; Tel.: +30-27210-45271 Abstract: Ergot alkaloids (EAs) are a group of mycotoxins that are mainly produced from the plant pathogen Claviceps. Claviceps purpurea is one of the most important species, being a major producer of EAs that infect more than 400 species of monocotyledonous plants. Rye, barley, wheat, millet, oats, and triticale are the main crops affected by EAs, with rye having the highest rates of fungal infection. The 12 major EAs are ergometrine (Em), ergotamine (Et), ergocristine (Ecr), ergokryptine (Ekr), ergosine (Es), and ergocornine (Eco) and their epimers ergotaminine (Etn), egometrinine (Emn), egocristinine (Ecrn), ergokryptinine (Ekrn), ergocroninine (Econ), and ergosinine (Esn). Given that many food products are based on cereals (such as bread, pasta, cookies, baby food, and confectionery), the surveillance of these toxic substances is imperative. Although acute mycotoxicosis by EAs is rare, EAs remain a source of concern for human and animal health as food contamination by EAs has recently increased. Environmental conditions, such as low temperatures and humid weather before and during flowering, influence contamination agricultural products by EAs, contributing to the Citation: Agriopoulou, S. Ergot Alkaloids Mycotoxins in Cereals and appearance of outbreak after the consumption of contaminated products. The present work aims to Cereal-Derived Food Products: present the recent advances in the occurrence of EAs in some food products with emphasis mainly Characteristics, Toxicity, Prevalence, on grains and grain-based products, as well as their toxicity and control strategies. -
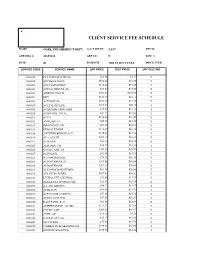
Oakland Sheriff's Dept Fee Schedule 02.09
CLIENT SERVICE FEE SCHEDULE NAME: OAKLAND SHERIFF'S DEPT ACCT SETUP: 2/1/87 REP ID: GRP BILL #: 22857610 GRP CD: N DISC %: FS ID: 01 FS DESCR: 2003 CLIENT FEES PRICE TYPE: SERVICE CODE SERVICE NAME UPF PRICE TEST PRICE UPF DISC IND 0000018 PLT SODIUM CITRATE $12.86 $4.37 Y 0000022 APC RESISTANCE $101.62 $34.55 Y 0000201 ACETAMINOPHEN $110.24 $37.48 Y 0000202 ACETALDEHYDE (B) $73.00 $73.00 N 0000203 ARSENIC,TISSUE $302.00 $302.00 N 0000204 DHT $182.70 $62.12 Y 0000205 ACETONE (B) $102.20 $34.75 Y 0000206 ACETYLCHOLINE $165.00 $56.10 Y 0000208 ACID PHOS, PROS, IMM $35.60 $12.10 Y 0000210 ACID PHOS, TOTAL $37.22 $12.65 Y 0000211 ACTH $110.00 $37.40 Y 0000212 AMYLASE (U) $45.45 $15.45 Y 0000213 IMMUNOFIX, UR $67.39 $22.91 Y 0000214 ETHOSUXIMIDE $112.07 $38.10 Y 0000216 ANTITHROMBIN III ACT $170.46 $57.96 Y 0000219 ALA, QUANT $107.10 $36.41 Y 0000223 ALBUMIN $22.88 $22.88 N 0000224 ALBUMIN, CSF $36.25 $12.33 Y 0000225 CYCLIC AMP, UR $205.80 $69.97 Y 0000227 ALDOLASE $32.08 $10.91 Y 0000228 A-2-MACROGLOB. $78.75 $26.78 Y 0000229 ALDOSTERONE (U) $251.58 $85.54 Y 0000230 ALDOSTERONE $207.10 $70.41 Y 0000231 ALK PHOS ISOENZYMES $61.34 $20.86 Y 0000232 AFP, FLUID W/RFX $107.40 $36.52 Y 0000233 LEUKOCYTE ALK PHOS $34.60 $11.76 Y 0000234 ALKALINE PHOSPHATASE $22.88 $22.88 N 0000235 A-1-ANTITRYPSN $40.22 $13.67 Y 0000236 AMIKACIN $99.91 $33.97 Y 0000237 AFP,TUMOR (CHIRON) $53.86 $18.31 Y 0000238 AMINO ACID SCR $87.15 $29.63 Y 0000240 RAST PANEL #103 $62.00 $62.00 N 0000241 AMPHETAMINE GC/MS $111.70 $37.98 Y 0000242 CYCLIC AMP $205.80 $69.97 Y 0000243 AMYLASE $16.42 $5.58 Y 0000248 HACKBERRY IGE $35.40 $35.40 N 0000249 ANA W/RFX $55.00 $18.70 Y 0000250 *AMIKACIN,PEAK&TROUGH $199.83 $67.94 Y 0000251 ANDROSTENEDIONE $180.65 $61.42 Y 0000252 ANTI-DIUR. -

Cabergoline Monograph
Cabergoline DRUG NAME: Cabergoline SYNONYM(S): COMMON TRADE NAME(S): DOSTINEX® CLASSIFICATION: hormonal agent Special pediatric considerations are noted when applicable, otherwise adult provisions apply. MECHANISM OF ACTION: Cabergoline is a dopaminergic ergot derivative with longer lasting prolactin lowering activity than bromocriptine. Cabergoline may decrease hormone production and the size of prolactin-dependent pituitary adenomas1 by inhibiting the release and synthesis of prolactin from the anterior pituitary gland.2,3 The prolactin lowering effect is dose-related.2 PHARMACOKINETICS: Oral Absorption rapidly absorbed, unaffected by food Distribution widely distributed,4 time to peak 2-3 h, steady state achieved after 4 weeks cross blood brain barrier? yes volume of distribution no information found plasma protein binding 40-42% Metabolism extensive hepatic metabolism, primarily via hydrolysis with minimal CYP450 mediated metabolism; undergoes first-pass metabolism active metabolite(s) no information found inactive metabolite(s)4-6 eight metabolites including 6-allyl-8b-carboxy-ergoline Excretion primarily hepatic7 urine7 18-22%, <4% unchanged after 20 days feces7 60-72% after 20 days terminal half life 63-69 h, hyperprolactinemic patients: 79-115 h clearance no information found Sex no significant differences found7 Elderly no significant differences found7 Adapted from standard reference2 unless specified otherwise. USES: Primary uses: Other uses: *Pituitary tumours *Health Canada approved indication SPECIAL PRECAUTIONS: Contraindications2: -

Endophytic Fungi: Treasure for Anti-Cancerous Compounds
International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 8, Issue 8, 2016 Review Article ENDOPHYTIC FUNGI: TREASURE FOR ANTI-CANCEROUS COMPOUNDS ANAND DILIP FIRODIYAa*, RAJESH KUMAR TENGURIAb aCSRD, Peoples University, Bhopal 462037, Madhya Pradesh, India, bDepartment of Botany, Govt. PG College, Rajgarh 496551, Madhya Pradesh, India Email: [email protected] Received: 22 Apr 2016 Revised and Accepted: 20 June 2016 ABSTRACT Endophytic fungi that live asymptomatically inside the plant tissues have novel bioactive metabolites exhibiting a variety of biological activities, especially against cancer. This review highlights the research progress on the production of anticancer compounds by endophytic fungi from 1990- 2015. Anticancer activity is generally associated with the cytotoxicity of the compounds present in the endophytic fungi. The ubiquitous nature of endophytic fungi synthesise diverse chemicals with promising anticancer activity from either their original host or related species. Modification in fermentation parameters and genetic insight of endophytes may produce novel anti-cancerous compounds. Keywords: Cancer, Medicinal plants, Secondary metabolites © 2016 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/) INTRODUCTION endophytic fungi detectable by high-performance liquid chromate- graphy, nuclear magnetic resonance, mass spectrophotometer and The interest in the biogenic medicines has revived throughout the X-ray crystallography and its cytotoxicity of the bioactive world, as the increase in awareness of the health hazards and compounds against cancer cell lines. The compounds with potential toxicity associated with the random use of synthetic drugs and application were also considered in the selection of antitumor antibiotics [1]. -

Near the Himalayas, from Kashmir to Sikkim, at Altitudes the Catholic Inquisition, and the Traditional Use of These of up to 2700 Meters
Year of edition: 2018 Authors of the text: Marc Aixalà & José Carlos Bouso Edition: Alex Verdaguer | Genís Oña | Kiko Castellanos Illustrations: Alba Teixidor EU Project: New Approaches in Harm Reduction Policies and Practices (NAHRPP) Special thanks to collaborators Alejandro Ponce (in Peyote report) and Eduardo Carchedi (in Kambó report). TECHNICAL REPORT ON PSYCHOACTIVE ETHNOBOTANICALS Volumes I - II - III ICEERS International Center for Ethnobotanical Education Research and Service INDEX SALVIA DIVINORUM 7 AMANITA MUSCARIA 13 DATURA STRAMONIUM 19 KRATOM 23 PEYOTE 29 BUFO ALVARIUS 37 PSILOCYBIN MUSHROOMS 43 IPOMOEA VIOLACEA 51 AYAHUASCA 57 IBOGA 67 KAMBÓ 73 SAN PEDRO 79 6 SALVIA DIVINORUM SALVIA DIVINORUM The effects of the Hierba Pastora have been used by Mazatec Indians since ancient times to treat diseases and for divinatory purposes. The psychoactive compound Salvia divinorum contains, Salvinorin A, is the most potent naturally occurring psychoactive substance known. BASIC INFO Ska Pastora has been used in divination and healing Salvia divinorum is a perennial plant native to the Maza- rituals, similar to psilocybin mushrooms. Maria Sabina tec areas of the Sierra Madre Oriental Mountains of Mexi- told Wasson and Hofmann (the discoverers of its Mazatec co. Its habitat is tropical forests, where it grows between usage) that Salvia divinorum was used in times when the- 300 and 800 meters above sea level. It belongs to the re was a shortage of mushrooms. Some sources that have Lamiaceae family, and is mainly reproduced by cuttings done later feldwork point out that the use of S. divinorum since it rarely produces seeds. may be more widespread than originally believed, even in times when mushrooms were abundant. -

Hallucinogens: a Cause of Convulsive Ergot Psychoses
Loma Linda University TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works Loma Linda University Electronic Theses, Dissertations & Projects 6-1976 Hallucinogens: a Cause of Convulsive Ergot Psychoses Sylvia Dahl Winters Follow this and additional works at: https://scholarsrepository.llu.edu/etd Part of the Psychiatry Commons Recommended Citation Winters, Sylvia Dahl, "Hallucinogens: a Cause of Convulsive Ergot Psychoses" (1976). Loma Linda University Electronic Theses, Dissertations & Projects. 976. https://scholarsrepository.llu.edu/etd/976 This Thesis is brought to you for free and open access by TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works. It has been accepted for inclusion in Loma Linda University Electronic Theses, Dissertations & Projects by an authorized administrator of TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works. For more information, please contact [email protected]. ABSTRACT HALLUCINOGENS: A CAUSE OF CONVULSIVE ERGOT PSYCHOSES By Sylvia Dahl Winters Ergotism with vasoconstriction and gangrene has been reported through the centuries. Less well publicized are the cases of psychoses associated with convulsive ergotism. Lysergic acid amide a powerful hallucinogen having one.-tenth the hallucinogenic activity of LSD-25 is produced by natural sources. This article attempts to show that convulsive ergot psychoses are mixed psychoses caused by lysergic acid amide or similar hallucinogens combined with nervous system -

And Mushroom-Associated Alkaloids from Two Behavior Modifying
bioRxiv preprint doi: https://doi.org/10.1101/375105; this version posted December 18, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Psychoactive plant- and mushroom-associated alkaloids from two behavior modifying 2 cicada pathogens 3 4 Greg R. Boyce1, Emile Gluck-Thaler2, Jason C. Slot2, Jason E. Stajich3, William J. Davis4, Tim 5 Y. James4, John R. Cooley5, Daniel G. Panaccione1, Jørgen Eilenberg6, Henrik H. De Fine Licht6, 6 Angie M. Macias1, Matthew C. Berger1, Kristen L. Wickert1, Cameron M. Stauder1, Ellie J. 7 Spahr1, Matthew D. Maust1, Amy M. Metheny1, Chris Simon5, Gene Kritsky7, Kathie T. Hodge8, 8 Richard A. Humber8,9, Terry Gullion10, Dylan P. G. Short11, Teiya Kijimoto1, Dan Mozgai12, 9 Nidia Arguedas13, Matt T. Kasson1,* 10 11 1Division of Plant and Soil Sciences, West Virginia University, Morgantown, West Virginia, 12 26506, USA. 13 2Department of Plant Pathology, The Ohio State University, Columbus, Ohio 43210, USA. 14 3Department of Microbiology and Plant Pathology and Institute for Integrative Genome Biology, 15 University of California, Riverside, California 92521, USA. 16 4Department of Ecology and Evolution, University of Michigan, Ann Arbor, Michigan 48109, 17 USA. 18 5Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, 19 Connecticut 06269, USA. 20 6Department of Plant and Environmental Science, University of Copenhagen, Denmark. 21 7Department of Biology, Mount St. Joseph University, Cincinnati, Ohio 45233, USA. 22 8Plant Pathology & Plant-Microbe Biology, School of Integrative Plant Science, Cornell 23 University, Ithaca, New York 14853, USA. -

Ergot Alkaloid Biosynthesis in Aspergillus Fumigatus : Association with Sporulation and Clustered Genes Common Among Ergot Fungi
Graduate Theses, Dissertations, and Problem Reports 2009 Ergot alkaloid biosynthesis in Aspergillus fumigatus : Association with sporulation and clustered genes common among ergot fungi Christine M. Coyle West Virginia University Follow this and additional works at: https://researchrepository.wvu.edu/etd Recommended Citation Coyle, Christine M., "Ergot alkaloid biosynthesis in Aspergillus fumigatus : Association with sporulation and clustered genes common among ergot fungi" (2009). Graduate Theses, Dissertations, and Problem Reports. 4453. https://researchrepository.wvu.edu/etd/4453 This Dissertation is protected by copyright and/or related rights. It has been brought to you by the The Research Repository @ WVU with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you must obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Dissertation has been accepted for inclusion in WVU Graduate Theses, Dissertations, and Problem Reports collection by an authorized administrator of The Research Repository @ WVU. For more information, please contact [email protected]. Ergot alkaloid biosynthesis in Aspergillus fumigatus: Association with sporulation and clustered genes common among ergot fungi Christine M. Coyle Dissertation submitted to the Davis College of Agriculture, Forestry, and Consumer Sciences at West Virginia University in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Genetics and Developmental Biology Daniel G. Panaccione, Ph.D., Chair Kenneth P. Blemings, Ph.D. Joseph B. -

Ergot Poisoning G
Postgrad Med J: first published as 10.1136/pgmj.42.491.562 on 1 September 1966. Downloaded from POSTGRAD. MED. J. (1966), 42, 562. Case Reports ERGOT POISONING G. GLAZER, M.B., B.S. K. A. MYERS, M.B.(Melb.), F.R.A.C.S. House Surgeon, Surgical Unit. Senior Registrar, Surgical Unit (Smith and Nephew Fellow). E. R. DAVIES, M.B., M.R.C.P.E., F.F.R. Senior Registrar, Radiological Dept., St. Mary's Hospital, London, W.2. ERGOTAMINE tartrate is frequently prescribed for the Progress: Following admission the patient became treatment of migraine. Complications from the drug drowsy, nauseated and suffered from attacks of vertigo. are rare but potentially serious. Five days after cessation of ergotamine therapy the foot A case is presented of severe lower limb arterial pulses became palpable and he then developed intense spasm due to ergotamine tartrate taken sublingually and burning sensations in both feet (St. Anthony's Fire). orally for migraine. This case provided an opportunity It was considered that final confirmation of the to study the vascular and systemic effects of the drug and diagnosis necessitated reproduction of the symptoms the literature the manifes- with a small provocative dose of ergotamine. One day to review concerning risk, after the return of the foot pulses, ergotamine tartrate tations and treatment of ergot poisoning. was recommenced, and a total of 10 mg. was given orally Case Report over a period of three days; the foot pulses disappeared Mr. E.K., a 33 year old van-driver, presented in eighteen hours after the initial 2 mg. -

Risk Assessment of Argyreia Nervosa
Risk assessment of Argyreia nervosa RIVM letter report 2019-0210 W. Chen | L. de Wit-Bos Risk assessment of Argyreia nervosa RIVM letter report 2019-0210 W. Chen | L. de Wit-Bos RIVM letter report 2019-0210 Colophon © RIVM 2020 Parts of this publication may be reproduced, provided acknowledgement is given to the: National Institute for Public Health and the Environment, and the title and year of publication are cited. DOI 10.21945/RIVM-2019-0210 W. Chen (author), RIVM L. de Wit-Bos (author), RIVM Contact: Lianne de Wit Department of Food Safety (VVH) [email protected] This investigation was performed by order of NVWA, within the framework of 9.4.46 Published by: National Institute for Public Health and the Environment, RIVM P.O. Box1 | 3720 BA Bilthoven The Netherlands www.rivm.nl/en Page 2 of 42 RIVM letter report 2019-0210 Synopsis Risk assessment of Argyreia nervosa In the Netherlands, seeds from the plant Hawaiian Baby Woodrose (Argyreia nervosa) are being sold as a so-called ‘legal high’ in smart shops and by internet retailers. The use of these seeds is unsafe. They can cause hallucinogenic effects, nausea, vomiting, elevated heart rate, elevated blood pressure, (severe) fatigue and lethargy. These health effects can occur even when the seeds are consumed at the recommended dose. This is the conclusion of a risk assessment performed by RIVM. Hawaiian Baby Woodrose seeds are sold as raw seeds or in capsules. The raw seeds can be eaten as such, or after being crushed and dissolved in liquid (generally hot water). -

Regulation of Alkaloid Biosynthesis in Plants
CONTRIBUTORS Numbers in parentheses indicate the pages on which the authors’ contributions begin. JAUME BASTIDA (87), Departament de Productes Naturals, Facultat de Farma` cia, Universitat de Barcelona, 08028 Barcelona, Spain YEUN-MUN CHOO (181), Department of Chemistry, University of Malaya, 50603 Kuala Lumpur, Malaysia PETER J. FACCHINI (1), Department of Biological Sciences, University of Calgary, Calgary, AB, Canada TOH-SEOK KAM (181), Department of Chemistry, University of Malaya, 50603 Kuala Lumpur, Malaysia RODOLFO LAVILLA (87), Parc Cientı´fic de Barcelona, Universitat de Barcelona, 08028 Barcelona, Spain DANIEL G. PANACCIONE (45), Division of Plant and Soil Sciences, West Virginia University, Morgantown, WV 26506-6108, USA CHRISTOPHER L. SCHARDL (45), Department of Plant Pathology, University of Kentucky, Lexington, KY 40546-0312, USA PAUL TUDZYNSKI (45), Institut fu¨r Botanik, Westfa¨lische Wilhelms Universita¨tMu¨nster, Mu¨nster D-48149, Germany FRANCESC VILADOMAT (87), Departament de Productes Naturals, Facultat de Farma` cia, Universitat de Barcelona, 08028 Barcelona, Spain vii PREFACE This volume of The Alkaloids: Chemistry and Biology is comprised of four very different chapters; a reflection of the diverse facets that comprise the study of alkaloids today. As awareness of the global need for natural products which can be made available as drugs on a sustainable basis increases, so it has become increas- ingly important that there is a full understanding of how key metabolic pathways can be optimized. At the same time, it remains important to find new biologically active alkaloids and to elucidate the mechanisms of action of those that do show potentially useful or novel biological effects. Facchini, in Chapter 1, reviews the significant studies that have been conducted with respect to how the formation of alkaloids in their various diverse sources are regulated at the molecular level. -
Fungal Endophytes As Efficient Sources of Plant-Derived Bioactive
microorganisms Review Fungal Endophytes as Efficient Sources of Plant-Derived Bioactive Compounds and Their Prospective Applications in Natural Product Drug Discovery: Insights, Avenues, and Challenges Archana Singh 1,2, Dheeraj K. Singh 3,* , Ravindra N. Kharwar 2,* , James F. White 4,* and Surendra K. Gond 1,* 1 Department of Botany, MMV, Banaras Hindu University, Varanasi 221005, India; [email protected] 2 Department of Botany, Institute of Science, Banaras Hindu University, Varanasi 221005, India 3 Department of Botany, Harish Chandra Post Graduate College, Varanasi 221001, India 4 Department of Plant Biology, Rutgers University, New Brunswick, NJ 08901, USA * Correspondence: [email protected] (D.K.S.); [email protected] (R.N.K.); [email protected] (J.F.W.); [email protected] (S.K.G.) Abstract: Fungal endophytes are well-established sources of biologically active natural compounds with many producing pharmacologically valuable specific plant-derived products. This review details typical plant-derived medicinal compounds of several classes, including alkaloids, coumarins, flavonoids, glycosides, lignans, phenylpropanoids, quinones, saponins, terpenoids, and xanthones that are produced by endophytic fungi. This review covers the studies carried out since the first report of taxol biosynthesis by endophytic Taxomyces andreanae in 1993 up to mid-2020. The article also highlights the prospects of endophyte-dependent biosynthesis of such plant-derived pharma- cologically active compounds and the bottlenecks in the commercialization of this novel approach Citation: Singh, A.; Singh, D.K.; Kharwar, R.N.; White, J.F.; Gond, S.K. in the area of drug discovery. After recent updates in the field of ‘omics’ and ‘one strain many Fungal Endophytes as Efficient compounds’ (OSMAC) approach, fungal endophytes have emerged as strong unconventional source Sources of Plant-Derived Bioactive of such prized products.