Phasmida: Phasmatidae)
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Ecomorph Convergence in Stick Insects (Phasmatodea) with Emphasis on the Lonchodinae of Papua New Guinea
Brigham Young University BYU ScholarsArchive Theses and Dissertations 2018-07-01 Ecomorph Convergence in Stick Insects (Phasmatodea) with Emphasis on the Lonchodinae of Papua New Guinea Yelena Marlese Pacheco Brigham Young University Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the Life Sciences Commons BYU ScholarsArchive Citation Pacheco, Yelena Marlese, "Ecomorph Convergence in Stick Insects (Phasmatodea) with Emphasis on the Lonchodinae of Papua New Guinea" (2018). Theses and Dissertations. 7444. https://scholarsarchive.byu.edu/etd/7444 This Thesis is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Ecomorph Convergence in Stick Insects (Phasmatodea) with Emphasis on the Lonchodinae of Papua New Guinea Yelena Marlese Pacheco A thesis submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Master of Science Michael F. Whiting, Chair Sven Bradler Seth M. Bybee Steven D. Leavitt Department of Biology Brigham Young University Copyright © 2018 Yelena Marlese Pacheco All Rights Reserved ABSTRACT Ecomorph Convergence in Stick Insects (Phasmatodea) with Emphasis on the Lonchodinae of Papua New Guinea Yelena Marlese Pacheco Department of Biology, BYU Master of Science Phasmatodea exhibit a variety of cryptic ecomorphs associated with various microhabitats. Multiple ecomorphs are present in the stick insect fauna from Papua New Guinea, including the tree lobster, spiny, and long slender forms. While ecomorphs have long been recognized in phasmids, there has yet to be an attempt to objectively define and study the evolution of these ecomorphs. -

Insecta: Phasmatodea) and Their Phylogeny
insects Article Three Complete Mitochondrial Genomes of Orestes guangxiensis, Peruphasma schultei, and Phryganistria guangxiensis (Insecta: Phasmatodea) and Their Phylogeny Ke-Ke Xu 1, Qing-Ping Chen 1, Sam Pedro Galilee Ayivi 1 , Jia-Yin Guan 1, Kenneth B. Storey 2, Dan-Na Yu 1,3 and Jia-Yong Zhang 1,3,* 1 College of Chemistry and Life Science, Zhejiang Normal University, Jinhua 321004, China; [email protected] (K.-K.X.); [email protected] (Q.-P.C.); [email protected] (S.P.G.A.); [email protected] (J.-Y.G.); [email protected] (D.-N.Y.) 2 Department of Biology, Carleton University, Ottawa, ON K1S 5B6, Canada; [email protected] 3 Key Lab of Wildlife Biotechnology, Conservation and Utilization of Zhejiang Province, Zhejiang Normal University, Jinhua 321004, China * Correspondence: [email protected] or [email protected] Simple Summary: Twenty-seven complete mitochondrial genomes of Phasmatodea have been published in the NCBI. To shed light on the intra-ordinal and inter-ordinal relationships among Phas- matodea, more mitochondrial genomes of stick insects are used to explore mitogenome structures and clarify the disputes regarding the phylogenetic relationships among Phasmatodea. We sequence and annotate the first acquired complete mitochondrial genome from the family Pseudophasmati- dae (Peruphasma schultei), the first reported mitochondrial genome from the genus Phryganistria Citation: Xu, K.-K.; Chen, Q.-P.; Ayivi, of Phasmatidae (P. guangxiensis), and the complete mitochondrial genome of Orestes guangxiensis S.P.G.; Guan, J.-Y.; Storey, K.B.; Yu, belonging to the family Heteropterygidae. We analyze the gene composition and the structure D.-N.; Zhang, J.-Y. -

The New Stick Insect Genus Pterulina Gen. Nov., a Second Winged
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/342530173 The new stick insect genus Pterulina gen. nov., a second winged Clitumninae genus from Vietnam with a new combination and a new species (Phasmida, Phasmatidae, Clitumninae, Clitumn... Article in Belgian Journal of Entomology · June 2020 CITATIONS READS 0 1,328 2 authors: Joachim Bresseel Jérôme Constant Royal Belgian Institute of Natural Sciences Royal Belgian Institute of Natural Sciences 28 PUBLICATIONS 140 CITATIONS 126 PUBLICATIONS 509 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Systematics of Philippine Auchenorrhyncha View project GLOBAL TAXONOMY INITIATIVE - Entomodiversity of VIETNAM View project All content following this page was uploaded by Jérôme Constant on 29 June 2020. The user has requested enhancement of the downloaded file. Belgian Journal of Entomology 96: 1–30 (2020) ISSN: 2295-0214 www.srbe-kbve.be urn:lsid:zoobank.org:pub:86A63507-90DC-445B-96A7-0BCA112D6C4D Belgian Journal of Entomology The new stick insect genus Pterulina gen. nov., a second winged Clitumninae genus from Vietnam with a new combination and a new species (Phasmida, Phasmatidae, Clitumninae, Clitumnini) Joachim BRESSEEL1 & Jérôme CONSTANT2 1, 2 Royal Belgian Institute of Natural Sciences, O.D. Phylogeny and Taxonomy, Entomology, Vautier street 29, B-1000 Brussels, Belgium 1 E-mail:[email protected] (corresponding author) urn:lsid:zoobank.org:author:3C4EF358-9716-46F0-8575-26BE1EDE4349 2 E-mail: [email protected] urn:lsid:zoobank.org:author:6E6072A1-9415-4C8D-8E60-2504444DB290 Published: Brussels, June 29, 2020 BRESSEEL J. -

Survival Meal for Medauroidea Extradentata (Brunner Von Wattenwyl 1907) (Phasmatodea: Phasmatidae) Gabriel Olive, Jean-Yves Zimmer, Gilles Olive
Mentha spicata var. spicata (L. 1753) and Raphanus sativus var. sativus (L. 1753) : survival meal for Medauroidea extradentata (Brunner von Wattenwyl 1907) (Phasmatodea: Phasmatidae) Gabriel Olive, Jean-Yves Zimmer, Gilles Olive To cite this version: Gabriel Olive, Jean-Yves Zimmer, Gilles Olive. Mentha spicata var. spicata (L. 1753) and Raphanus sativus var. sativus (L. 1753) : survival meal for Medauroidea extradentata (Brunner von Watten- wyl 1907) (Phasmatodea: Phasmatidae). Communications in Agricultural and Applied Biological Sciences, University Gent, 2016, 81 (1), pp.190-193. hal-01270314 HAL Id: hal-01270314 https://hal.archives-ouvertes.fr/hal-01270314 Submitted on 17 Feb 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 190 MENTHA SPICATA VAR. SPICATA (L. 1753) AND RAPHANUS SATIVUS VAR. SATIVUS (L. 1753): SURVIVAL MEAL FOR MEDAUROIDEA EXTRADENTATA (BRUNNER VON WATTENWYL 1907) (PHASMATODEA: PHASMATIDAE) GABRIEL OLIVE*, JEAN-YVES ZIMMER**, GILLES OLIVE* *Ecole Industrielle et Commerciale de la Ville de Namur, Laboratoire C2A, Rue Pépin, -

Downloaded and Searched Using
bioRxiv preprint doi: https://doi.org/10.1101/453514; this version posted November 17, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Title: Bacterial contribution to genesis of the novel germ line determinant oskar 2 3 Authors: Leo Blondel1, Tamsin E. M. Jones2,3 and Cassandra G. Extavour1,2* 4 5 Affiliations: 6 1. Department of Molecular and Cellular Biology, Harvard University, 16 Divinity Avenue, 7 Cambridge MA, USA 8 2. Department of Organismic and Evolutionary Biology, Harvard University, 16 Divinity 9 Avenue, Cambridge MA, USA 10 3. Current address: European Bioinformatics Institute, EMBL-EBI, Wellcome Genome 11 Campus, Hinxton, Cambridgeshire, UK 12 13 * Correspondence to [email protected] 14 15 Abstract: New cellular functions and developmental processes can evolve by modifying 16 existing genes or creating novel genes. Novel genes can arise not only via duplication or 17 mutation but also by acquiring foreign DNA, also called horizontal gene transfer (HGT). Here 18 we show that HGT likely contributed to the creation of a novel gene indispensable for 19 reproduction in some insects. Long considered a novel gene with unknown origin, oskar has 20 evolved to fulfil a crucial role in insect germ cell formation. Our analysis of over 100 insect 21 Oskar sequences suggests that Oskar arose de novo via fusion of eukaryotic and prokaryotic 22 sequences. This work shows that highly unusual gene origin processes can give rise to novel 23 genes that can facilitate evolution of novel developmental mechanisms. -

The Stick Insect Genus Medauroidea Zompro, 2000: Taxonomic Note and Extension to Laos and Cambodia with One New Species, M
Belgian Journal of Entomology 73: 1–19 (2018) ISSN: 2295-0214 www.srbe-kbve.be urn:lsid:zoobank.org:pub:09D65188-1D26-4A2C-8B9D-C44FA7B29F4E Belgian Journal of Entomology The stick insect genus Medauroidea Zompro, 2000: Taxonomic note and extension to Laos and Cambodia with one new species, M. romantica sp. nov. (Phasmida: Phasmatidae: Clitumninae) Joachim BRESSEEL¹ & Jérôme CONSTANT² 1,2 Royal Belgian Institute of Natural Sciences, O.D. Phylogeny and Taxonomy, Entomology, Vautier street 29, B-1000 Brussels, Belgium 1 E-mail:[email protected] (corresponding author) urn:lsid:zoobank.org:author:3C4EF358-9716-46F0-8575-26BE1EDE4349 2 E-mail: [email protected] urn:lsid:zoobank.org:author:6E6072A1-9415-4C8D-8E60-2504444DB290 Published: Brussels, July 09, 2018 Citation: BRESSEEL J. & CONSTANT J., 2018 - The stick insect genus Medauroidea Zompro, 2000: Taxonomic note and extension to Laos and Cambodia with one new species, M. romantica sp. nov. (Phasmida: Phasmatidae: Clitumninae). Belgian Journal of Entomology, 73: 1–19. ISSN: 1374-5514 (Print Edition) ISSN: 2295-0214 (Online Edition) The Belgian Journal of Entomology is published by the Royal Belgian Society of Entomology, a non-profit association established on April 9, 1855. Head office: Vautier street 29, B-1000 Brussels. The publications of the Society are partly sponsored by the University Foundation of Belgium. In compliance with Article 8.6 of the ICZN, printed versions of all papers are deposited in the following libraries: - Royal Library of Belgium, Boulevard de l’Empereur 4, B-1000 Brussels. - Library of the Royal Belgian Institute of Natural Sciences, Vautier street 29, B-1000 Brussels. -

Research.Pdf (1.406Mb)
INVESTIGATING AN ADAPTIVE RADIATION IN TEMPERATE NEOCONOCEPHALUS _______________________________________ A Dissertation presented to the Faculty of the Graduate School University of Missouri-Columbia _______________________________________________________ In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy _____________________________________________________ by Katherine H. Frederick Dr. Johannes Schul, Dissertation Supervisor JULY 2013 The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled INVESTIGATING AN ADAPTIVE RADIATION IN TEMPERATE NEOCONOCEPHALUS Presented by Katherine H. Frederick, A candidate for the degree of Doctor of Philosophy And hereby certify that, in their opinion, it is worthy of acceptance. Professor Johannes Schul Professor Sarah L. Bush Professor Lori Eggert Professor Richard Houseman Professor J. Chris Pires ACKNOWLEDGEMENTS I would like to acknowledge all past and present Schul lab members, as well as all of the scientists that have contributed to the field of knowledge of Necoconocephalus acoustic communication; the time and effort of each of them contributed a data point or many points for use in comparative methods. I would like to thank Katie Kilmer, Oli Beckers, Gideon Ney, Nathan Thompson, and Megan Murphy for their help collecting specimens. I would like to thank Rob Snyder for his guidance in sequencing and tree building. Finally, I would like to thank Kate Hertweck, Roxi Kellar and Corey Hudson for their help with data analysis and discussions of evolutionary theory. Most importantly I would like to thank Johannes Schul and Sarah Bush. The Schul lab environment allowed me to bring knowledge and ideas from my previous studies and build on it in ways I never could have predicted. -
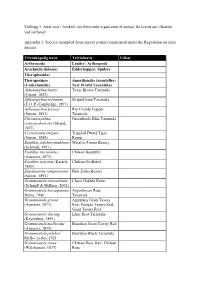
List of Species Exempted from Import Permit Requirement Pdf, 133.2
Vedlegg 1. Arter som i forskrift om fremmede organismer er unntatt fra kravet om tillatelse ved innførsel: Appendix 1. Species exempted from import permit requirement under the Regulation on alien species: Vitenskapelig navn Trivialnavn Vilkår Arthropoda Leddyr; Arthropods Arachnida (Klasse) Edderkopper; Spiders Theraphosidae Theraposinae Amerikanske taranteller; (Underfamilie) New World Tarantulas Aphonopelma hentzi Texas Brown Tarantula (Girard, 1852) Aphonopelma seemanni Striped-knee Tarantula (F.O. P.-Cambridge, 1897) Aphonopelma texense Rio Grande Copper (Simon, 1891) Tarantula Chromatopelma Greenbottle Blue Tarantula cyaneopubescens (Strand, 1907) Cyriocosmus elegans Trinidad Dwarf Tiger (Simon, 1889) Rump Euathlus pulcherrimaklaasi Metallic Femur Beauty (Schmidt, 1991) Euathlus truculentus Chilean Beautiful (Ausserer, 1875) Euathlus vulpinus (Karsch, Chilean Ocellated 1880) Eupalaestrus campestratus Pink Zebra Beauty (Simon, 1891) Grammostola aureostriata Chaco Golden Knee (Schmidt & Bullmer, 2001) Grammostola burzaquensis Argentinean Rose Ibarra, 1946 Tarantula Grammostola grossa Argentina Giant Tawny (Ausserer, 1871) Red, Pampas Tawny Red, Giant Tawny Red Grammostola iheringi Entre Rios Tarantula (Keyserling, 1891) Grammostola mollicoma Brazilian Giant Tawny Red (Ausserer, 1875) Grammostola pulchra Brazilian Black Tarantula Mello- Leitao, 1921 Grammostola rosea Chilean Rose Hair, Chilean (Walckenaer, 1837) Rose Vitenskapelig navn Trivialnavn Vilkår Lasiodora difficilis Mello- Fiery Redrump Leitao, 1921 Lasiodora klugi (C.L. Baja -

The Phasmid Study Group Newsletter No
The Phasmid Study Group Newsletter No. 109 March 2007 ISSN 0268-3806 . Includes Culture List Left: Macynia mcgregororum ♀, from ‘Following in Thunberg’s Footsteps’ Above Right: Peruphasma schultei (♀) mating with Pseudophasma rufipes (♂) [Courtesy of Mieke Duytschaever] Below Right: Mike Smith’s winning photograph of Extatosoma tiaratum (♀) News, Information & Updates ........................................................................................................................................................................ 3 Editorial.................................................................................................................................................................................................... 3 Diary Dates .............................................................................................................................................................................................. 3 Sticks In The News................................................................................................................................................................................... 4 PSG Committee changes (January 2007) ................................................................................................................................................ 4 Phasmid Studies ...................................................................................................................................................................................... 5 The Phasmida Species -
Phasmid Studies
ISSN 09660011 PHASMID STUDIES. volume 4, numbers 1 & 2. June & December 1995. Editor: P.E. Bragg. Published by the Phasmid Study Group. Phasmid Studies ISSN 0966-0011 volume 4, numbers 1 & 2. Contents Phasmids in the Western Australia Museum P.E. Bragg . A new (hot) method of collecting stick insects in Australia Lyn Lowe & Paul Brock . .. 2 The distribution of Aretaon in Borneo P.E. Bragg 4 A survey of the phasmids held in the collection of the National Museum of Wales, Cathays Park , Cardiff Stephen CrosbyClark . 6 Type species of phasmid genera with particular reference to the status of Baculum Saussure, 1861, Ramulus Saussure , 1862, and Gratidia Stal, 1875 . P.E. Bragg 11 A survey into the distribution of the stick insects of Britain Malcolm Lee 15 Comments on the species of Phasmida described by Stoll in 1788 and named by Olivier in 1792 P.E. Bragg 24 Reviews and Abstracts Book Reviews 26 Phasmid Abstracts . 27 Obituary 32 Food plants for PhyL!ium bioculatum Gray in Sri Lanka Charles Woolman & Haris Dharmasiri . 33 A short description of some deformed eggs of BaciLLus lynceorum lngo Fritzsche 37 Baculum sp. from Chiang Mai (PSG 153) Wim Potvin . .. 39 Water balance in phasmids and other insects R. Bradburne . .. 43 The culture of Bornean phasmids P.E. Bragg 50 Polyphagy in Clonopsis gaLlica (Charpentier): a survey of woody plant species Michael G. Guye . 54 The survival of newlyhatched leaf insects Amelia Hanibeltz, Yoko Nakamura, Alison lmms, and Eva Abdullah . .. 60 PSG 72, PhyLLium giganteum Hausleithner Frank Hennemann . .. 64 PSG 89 , an unidentified species of Necrosciinae Frank Hennemann . -

Report of Activities 2013
DGD-RBINS programmeme Protocol of cooperation REPORT 2013 Building capacities for biodiversity and development Burkina [email protected]. Susini Document prepared by L. Janssens de Bisthoven , H. de Koeijer, J. Constant, T. Delsinne, W. Dekoninck, Z. Nagy, P. Grootaert, F. Muhashy Habiyaremye, P. Luyten, K. Baetens, V. Pinton, Y. Samyn, M-L. Susini, E. Verheyen, and K. Vrancken. Benin © M.-L. Susini/RBINS Acronyms ABS Access and Benefit Sharing BTC Belgian Technical Cooperation CBD Convention on Biological Diversity CHM Clearing House Mechanism CITES Convention on International Trade in Endangered Species of wild fauna and flora CNEDD Conseil National de l’Environnement pour un Développement Durable, Niger COHERENS Coupled Hydrodynamic Ecological Model for Regional Shelf Seas COMIFAC Commission des Forêts d’Afrique Centrale COORD Programme Coordination and Management COP Conference of the Parties CSB Centre de Surveillance de la Biodiversité DGD Belgian Development Cooperation EDIT European Distributed Institute of Taxonomy GTI Global Taxonomy Initiative ICCN Institut Congolais pour la Conservation de la Nature, Kinshasa, D.R. Congo ICT Information and Computer Technology IEBR Institute of Ecology and Biological Resources, Hanoi, Viet Nam IFS International Foundation for Science, Sweden IMAB Inventories Monitoring and Assessment of Biodiversity Institut National pour l’Environnement et la Conservation de la Nature, Bujumbura, INECN Burundi Institut Supérieur de Conservation de la Nature, de l’Environnement et du Tourisme , ISCNET R.D. Congo ISDR-GL -

Phasmida, Phasmatidae, Clitumninae)
Belgian Journal of Entomology 76: 1–11 (2018) ISSN: 2295-0214 www.srbe-kbve.be urn:lsid:zoobank.org:pub:34E2ECC5-5E63-45CA-B128-D39CA5D77491 Belgian Journal of Entomology New synonymy and new records in the stick insect genus Medauromorpha Bresseel & Constant, 2017 (Phasmida, Phasmatidae, Clitumninae) Jérôme CONSTANT¹ & Joachim BRESSEEL² 1,2 Royal Belgian Institute of Natural Sciences, O.D. Phylogeny and Taxonomy, Entomology, Vautier street 29, B-1000 Brussels, Belgium. 1 urn:lsid:zoobank.org:author:6E6072A1-9415-4C8D-8E60-2504444DB290 1 Email: [email protected] 2 urn:lsid:zoobank.org:author:3C4EF358-9716-46F0-8575-26BE1EDE4349 2 Email: [email protected] (corresponding author) Published: Brussels, October 25, 2018 Citation: CONSTANT J. & BRESSEEL J., 2018. - New synonymy and new records in the stick insect genus Medauromorpha Bresseel & Constant, 2017 (Phasmida, Phasmatidae, Clitumninae). Belgian Journal of Entomology, 76: 1–11. ISSN: 1374-5514 (Print Edition) ISSN: 2295-0214 (Online Edition) The Belgian Journal of Entomology is published by the Royal Belgian Society of Entomology, a non-profit association established on April 9, 1855. Head office: Vautier street 29, B-1000 Brussels. The publications of the Society are partly sponsored by the University Foundation of Belgium. In compliance with Article 8.6 of the ICZN, printed versions of all papers are deposited in the following libraries: - Royal Library of Belgium, Boulevard de l’Empereur 4, B-1000 Brussels. - Library of the Royal Belgian Institute of Natural Sciences, Vautier street 29, B-1000 Brussels. - American Museum of Natural History Library, Central Park West at 79th street, New York, NY 10024-5192, USA.