Life Cycle of Early Cambrian Microalgae from the Skiagia
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Evolution of Genetic Systems in Filamentous Ascomycetes
Evolution of Genetic Systems in Filamentous Ascomycetes Evolutie van genetische systemen in hyphenvormende zakjeszwammen 0000 0513 3836 Promotor: dr. R.F. Hoekstra hoogleraar in de populatie- en kwantitatieve genetica fjtfoiißi f ßin Maarten J. Nauta Evolution of Genetic Systems in Filamentous Ascomycetes Proefschrift ter verkrijging van de graad van doctor in de landbouw- en milieuwetenschappen op gezag van de rector magnificus, dr. C.M. Karssen, in het openbaar te verdedigen op woensdag 12januar i 1994 des namiddags te vier uur in de Aula van de Landbouwuniversiteit te Wageningen. 15 0 S(p^ZJ> These investigations were supported by the Netherlands Organization for Scientific Research (N.W.O.). BibUt/FHEEK LAMDbOirWUNIVERSITEJi. WAGE NINGE N CIP-GEGEVENS KONINKLIJKE BIBLIOTHEEK, DEN HAAG Nauta, Maarten J. Evolution of genetic systems in filamentous ascomycetes / Maarten J. Nauta. - [ S.l. : s.n.]. -111 . Thesis Wageningen. - With ref. - With summary in Dutch. ISBN 90-5485-199-6 Subject headings: population genetics / ascomycetes. omslagontwerp: Ernst van Cleef foto omslag: Barrages tussen verschillende stammen van Podospora anserina als gevolg van vegetatieve incompatibiliteit. (met dank aan Inge Haspels) aan mijn ouders Voorwoord Dit proefschrift is het resultaat van vier jaar onderzoek, verricht bij de vakgroep Erfelijkheidsleer van de Landbouwuniversiteit in Wageningen. In zekere zin valt zo'n proefschrift te vergelijken met een levend wezen. Uit de genetica is bekend dat de verschijningsvorm van elk levend wezen tot stand komt door een combinatie van erfelijke aanleg en invloeden uit de omgeving. Voor een proefschrift geldt eigenlijk hetzelfde: Zowel het werk van de auteur, als de bijdragen van zijn omgeving zijn onontbeerlijk om tot een verschijningsvorm te komen. -

The Hawaiian Freshwater Algae Biodiversity Survey
Sherwood et al. BMC Ecology 2014, 14:28 http://www.biomedcentral.com/1472-6785/14/28 RESEARCH ARTICLE Open Access The Hawaiian freshwater algae biodiversity survey (2009–2014): systematic and biogeographic trends with an emphasis on the macroalgae Alison R Sherwood1*, Amy L Carlile1,2, Jessica M Neumann1, J Patrick Kociolek3, Jeffrey R Johansen4, Rex L Lowe5, Kimberly Y Conklin1 and Gernot G Presting6 Abstract Background: A remarkable range of environmental conditions is present in the Hawaiian Islands due to their gradients of elevation, rainfall and island age. Despite being well known as a location for the study of evolutionary processes and island biogeography, little is known about the composition of the non-marine algal flora of the archipelago, its degree of endemism, or affinities with other floras. We conducted a biodiversity survey of the non-marine macroalgae of the six largest main Hawaiian Islands using molecular and microscopic assessment techniques. We aimed to evaluate whether endemism or cosmopolitanism better explain freshwater algal distribution patterns, and provide a baseline data set for monitoring future biodiversity changes in the Hawaiian Islands. Results: 1,786 aquatic and terrestrial habitats and 1,407 distinct collections of non-marine macroalgae were collected from the islands of Kauai, Oahu, Molokai, Maui, Lanai and Hawaii from the years 2009–2014. Targeted habitats included streams, wet walls, high elevation bogs, taro fields, ditches and flumes, lakes/reservoirs, cave walls and terrestrial areas. Sites that lacked freshwater macroalgae were typically terrestrial or wet wall habitats that were sampled for diatoms and other microalgae. Approximately 50% of the identifications were of green algae, with lesser proportions of diatoms, red algae, cyanobacteria, xanthophytes and euglenoids. -

Algal Sex Determination and the Evolution of Anisogamy James Umen, Susana Coelho
Algal Sex Determination and the Evolution of Anisogamy James Umen, Susana Coelho To cite this version: James Umen, Susana Coelho. Algal Sex Determination and the Evolution of Anisogamy. Annual Review of Microbiology, Annual Reviews, 2019, 73 (1), 10.1146/annurev-micro-020518-120011. hal- 02187088 HAL Id: hal-02187088 https://hal.sorbonne-universite.fr/hal-02187088 Submitted on 17 Jul 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Annu. Rev. Microbiol. 2019. 73:X–X https://doi.org/10.1146/annurev-micro-020518-120011 Copyright © 2019 by Annual Reviews. All rights reserved Umen • Coelho www.annualreviews.org • Algal Sexes and Mating Systems Algal Sex Determination and the Evolution of Anisogamy James Umen1 and Susana Coelho2 1Donald Danforth Plant Science Center, St. Louis, Missouri 63132, USA; email: [email protected] 2Sorbonne Université, UPMC Université Paris 06, CNRS, Algal Genetics Group, UMR 8227, Integrative Biology of Marine Models, Station Biologique de Roscoff, CS 90074, F-29688, Roscoff, France [**AU: Please write the entire affiliation in French or write it all in English, rather than a combination of English and French**] ; email: [email protected] Abstract Algae are photosynthetic eukaryotes whose taxonomic breadth covers a range of life histories, degrees of cellular and developmental complexity, and diverse patterns of sexual reproduction. -

JJB 079 255 261.Pdf
植物研究雑誌 J. J. Jpn. Bo t. 79:255-261 79:255-261 (2004) Phylogenetic Phylogenetic Analysis of the Tetrasporalean Genus Asterococcus Asterococcus (Chlorophyceae) sased on 18S 18S Ribosomal RNA Gene Sequences Atsushi Atsushi NAKAZA WA and Hisayoshi NOZAKI Department Department of Biological Sciences ,Graduate School of Science ,University of Tokyo , Hongo Hongo 7-3-1 ,Bunkyo-ku ,Tokyo ,113 ・0033 JAPAN (Received (Received on October 30 ,2003) Nucleotide Nucleotide sequences (1642 bp) from 18S ribosomal RNA genes were analyzed for 100 100 strains of the clockwise (CW) group of Chlorophyceae to deduce the phylogenetic position position of the immotile colonial genus Asterococcus Scherffel , which is classified in the Palmellopsidaceae Palmellopsidaceae of Tetrasporales. We found that the genus Asterococcus and two uni- cellular , volvocalean genera , Lobochlamys Proschold & al. and Oogamochlamys Proschold Proschold & al., formed a robust monophyletic group , which was separated from two te 位asporalean clades , one composed of Tetraspora Link and Paulschulzia Sk 吋a and the other other containing the other palme l1 0psidacean genus Chlamydocaps αFot t. Therefore , the Tetrasporales Tetrasporales in the CW group is clearly polyphyletic and taxonomic revision of the order order and the Palmellopsidaceae is needed. Key words: 18S rRNA gene ,Asterococcus ,Palmellopsidaceae ,phylogeny ,Tetraspor- ales. ales. Asterococcus Asterococcus Scherffel (1908) is a colo- Recently , Ettl and Gartner (1 988) included nial nial green algal genus that is characterized Asterococcus in the family Palmello- by an asteroid chloroplast in the cell and psidaceae , because cells of this genus have swollen swollen gelatinous layers surrounding the contractile vacuoles and lack pseudoflagella immotile immotile colony (e. g. -

Phylogenetic Placement of Botryococcus Braunii (Trebouxiophyceae) and Botryococcus Sudeticus Isolate Utex 2629 (Chlorophyceae)1
J. Phycol. 40, 412–423 (2004) r 2004 Phycological Society of America DOI: 10.1046/j.1529-8817.2004.03173.x PHYLOGENETIC PLACEMENT OF BOTRYOCOCCUS BRAUNII (TREBOUXIOPHYCEAE) AND BOTRYOCOCCUS SUDETICUS ISOLATE UTEX 2629 (CHLOROPHYCEAE)1 Hoda H. Senousy, Gordon W. Beakes, and Ethan Hack2 School of Biology, University of Newcastle upon Tyne, Newcastle upon Tyne NE1 7RU, UK The phylogenetic placement of four isolates of a potential source of renewable energy in the form of Botryococcus braunii Ku¨tzing and of Botryococcus hydrocarbon fuels (Metzger et al. 1991, Metzger and sudeticus Lemmermann isolate UTEX 2629 was Largeau 1999, Banerjee et al. 2002). The best known investigated using sequences of the nuclear small species is Botryococcus braunii Ku¨tzing. This organism subunit (18S) rRNA gene. The B. braunii isolates has a worldwide distribution in fresh and brackish represent the A (two isolates), B, and L chemical water and is occasionally found in salt water. Although races. One isolate of B. braunii (CCAP 807/1; A race) it grows relatively slowly, it sometimes forms massive has a group I intron at Escherichia coli position 1046 blooms (Metzger et al. 1991, Tyson 1995). Botryococcus and isolate UTEX 2629 has group I introns at E. coli braunii strains differ in the hydrocarbons that they positions 516 and 1512. The rRNA sequences were accumulate, and they have been classified into three aligned with 53 previously reported rRNA se- chemical races, called A, B, and L. Strains in the A race quences from members of the Chlorophyta, includ- accumulate alkadienes; strains in the B race accumulate ing one reported for B. -

Acidotropic Probes and Flow Cytometry: a Powerful Combination for Detecting Phagotrophy in Mixotrophic and Heterotrophic Protists
AQUATIC MICROBIAL ECOLOGY Vol. 44: 85–96, 2006 Published August 16 Aquat Microb Ecol Acidotropic probes and flow cytometry: a powerful combination for detecting phagotrophy in mixotrophic and heterotrophic protists Wanderson F. Carvalho*, Edna Granéli Marine Science Department, University of Kalmar, 391 82 Kalmar, Sweden ABSTRACT: Studies with phagotrophic organisms are hampered by a series of methodological con- straints. To overcome problems related to the detection and enumeration of mixotrophic and hetero- trophic cells containing food vacuoles, we combined flow cytometry and an acidotropic blue probe as an alternative method. Flow cytometry allows the analysis of thousands of cells per minute with high sensitivity to the autofluorescence of different groups of cells and to probe fluorescence. The method was first tested in a grazing experiment where the heterotrophic dinoflagellate Oxyrrhis marina fed on Rhodomonas salina. The maximum ingestion rate of O. marina was 1.7 prey ind.–1 h–1, and the fre- quency of cells with R. salina in the food vacuoles increased from 0 to 2.4 ± 0.5 × 103 cells ml–1 within 6 h. The blue probe stained 100% of O. marina cells that had R. salina in the food vacuoles. The acidotropic blue probe was also effective in staining food vacuoles in the mixotrophic dinoflagellate Dinophysis norvegica. We observed that 75% of the D. norvegica population in the aphotic zone pos- sessed food vacuoles. Overall, in cells without food vacuoles, blue fluorescence was as low as in cells that were kept probe free. Blue fluorescence in O. marina cells with food vacuoles was 6-fold higher than in those without food vacuoles (20 ± 4 and 3 ± 0 relative blue fluorescence cell–1, respectively), while in D. -

Antimicrobial Peptides Expression for Defense System in Chicken Gastrointestinal and Reproductive Organs
The 6th International Seminar on Tropical Animal Production Integrated Approach in Developing Sustainable Tropical Animal Production October 20-22, 2015, Yogyakarta, Indonesia Antimicrobial Peptides Expression for Defense System in Chicken Gastrointestinal and Reproductive Organs Yukinori Yoshimura1, 2 Bambang Ariyadi3 and Naoki Isobe1, 2 1Graduate School of Biosphere Science, Hiroshima University, Higashi-Hiroshima 739- 8528, Japan; 2Research Center for Animal Science, Hiroshima University, Higashi-Hiroshima 739-8528, Japan; 3Faculty of Animal Science, Universitas Gadjah Mada, Yogyakarta, 55281 Indonesia. Email address: [email protected] ABSTRACT: Maintenance of animal health is essential to obtain their maximum productivity and safe products. Avian β-defensins (AvBDs) are the member of antimicrobial peptides, and Toll-like receptors (TLRs) are the primary receptors that recognize pathogen-associated molecular patterns (PAMPs) of microbes. The aim of this study was to characterize the innate immune system with the focus on the expression of AvBDs in the gastrointestinal tract and reproductive organs for the strategy to enhance the disease resistance of chickens. The proventriculus and cecum of broiler chicks expressed TLRs and AvBDs. It is suggested that a variety of PAMPs of microbes are recognized by different TLRs, probably leading to regulate the synthesis of innate immune factors including AvBDs. In laying hens, TLRs and AvBDs were expressed in the theca and granulosa layers of ovarian follicles and in the oviduct. In vivo LPS challenge increased the expression of several AvBDs in the theca tissue. In contrast, in the cultured theca tissue, LPS upregulated the expression of IL1β and IL6, but did not affect the AvBDs expression; whereas IL1β upregulated the expression of the AvBD12 gene and protein. -

Sex in the Extremes: Lichen-Forming Fungi
Mycologist, Volume 19, Part 2 May 2005. ©Cambridge University Press Printed in the United Kingdom. DOI: 10.1017/S0269915XO5002016 Sex in the extremes: lichen-forming fungi FABIAN A. SEYMOUR, PETER D. CRITTENDEN & PAUL S. DYER* School of Biology, University of Nottingham, University Park, Nottingham, NG7 2RD, UK. Tel. +44 (0) 115 9513203, Fax +44 (0) 115 9513251 E-mail: [email protected]; [email protected] *Corresponding Author Lichens are characteristically found in environments subject to extremes of temperature, desiccation and low nutrient status. Despite this sexual structures are often formed in abundance. The underlying mechanisms of sex in lichen-forming fungi are discussed, together with possible ecological reasons for the persistence of sexuality. Special features of lichen sex are highlighted including sex at the limits of life on earth in Antarctica, re-licheniza- tion following sex and dispersal, and the perennial nature of lichen fruiting bodies. Keywords: lichen, fungi, sex, breeding system, (98%) belonging to the Ascomycotina (Kirk et al., symbiosis, extreme environments, Antarctica 2001). They display a variety of morphologies, from flattened crust (crustose) or leafy (foliose) forms to Lichens - living together in a long-term relation- shrubby or pendulous fruticose types (Honegger, 2001) ship (Figs 3, 4, 7, 8). Lichens are seen as a textbook example of a successful Life in extreme environments mutualistic symbiosis. They consist of at least two A key characteristic of lichens is that they have a organisms: a fungus (the ‘mycobiont’), and an remarkable ability to tolerate extreme environmental intimately associated photosynthetic partner (the conditions and sustain growth despite frequent cycles ‘photobiont’). -

Phytochemistry, Pharmacology and Agronomy of Medicinal Plants: Amburana Cearensis, an Interdisciplinary Study
17 Phytochemistry, Pharmacology and Agronomy of Medicinal Plants: Amburana cearensis, an Interdisciplinary Study Kirley M. Canuto, Edilberto R. Silveira, Antonio Marcos E. Bezerra, Luzia Kalyne A. M. Leal and Glauce Socorro B. Viana Empresa Brasileira de Pesquisa Agropecuária, Universidade Federal do Ceará, Brazil 1. Introduction Plants are an important source of biologically active substances, therefore they have been used for medicinal purposes, since ancient times. Plant materials are used as home remedies, in over-the-counter drug products, dietary supplements and as raw material for obtention of phytochemicals. The use of medicinal plants is usually based on traditional knowledge, from which their therapeutic properties are oftenly ratified in pharmacological studies. Nowadays, a considerable amount of prescribed drug is still originated from botanical sources and they are associated with several pharmacological activities, such as morphine (I) (analgesic), scopolamine (II) atropine (III) (anticholinergics), galantamine (IV) (Alzheimer's disease), quinine (V) (antimalarial), paclitaxel (VI), vincristine (VII) and vinblastine (VIII) (anticancer drugs), as well as with digitalis glycosides (IX) (heart failure) (Fig. 1). The versatility of biological actions can be attributed to the huge amount and wide variety of secondary metabolites in plant organisms, belonging to several chemical classes as alkaloids, coumarins, flavonoids, tannins, terpenoids, xanthones, etc. The large consumption of herbal drugs, in spite of the efficiency of -
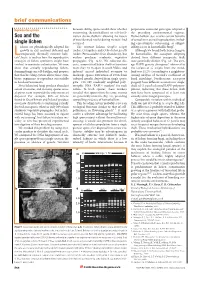
Brief Comms Layout 6/4Mx
brief communications Reproductive systems between sibling spores would show whether perpetuates successful genotypes adapted to outcrossing (heterothallism) or self-fertil- the prevailing environmental regimes. Sex and the ization (homothallism: allowing the fusion Homothallism also retains certain benefits of two identical nuclei during meiosis) had of sexual over asexual reproduction, includ- single lichen occurred. ing opportunistic outcrossing, as obligate ichens are physiologically adapted for The crustose lichens Graphis scripta selfing is rare in homothallic fungi8. growth in dry, nutrient-deficient and (order: Ostropales) and Ochrolechia parella Although we found both lichen fungi to Ltemporarily thermally extreme habi- (order: Pertusariales) fruit abundantly, but be homothallic, the ascospore offspring tats1, but it is unclear how the reproductive neither produces symbiotic vegetative derived from different conspecific thalli strategies of lichen symbionts might have propagules (Fig. 1a,b). We collected dis- were genetically distinct (Fig. 1d). The aver- evolved to maximize colonization. We now crete, symmetrical lichen thalli at locations age RAPD genetic divergence9 observed in show that sexually reproducing lichen- more than 10 m apart in south Wales, and five isolates of G. scripta from one wood- forming fungi can self-fertilize, and propose induced excised individual ascomata to land was 15.2% (according to a neighbour- that this breeding system allows these sym- discharge spores. Extraction of DNA from joining analysis of Jaccard’s coefficient of biotic organisms to reproduce successfully cultured mycelia derived from single spores band matching). Furthermore, ascospore in harsh environments. gave 218–263 randomly amplified poly- progeny from different ascomata on ‘single’ Most lichenized fungi produce abundant morphic DNA (RAPD) markers4 for each thalli of O. -

Chlorophyta, Chlorococcales, Oocystaceae) Outside East Asia
Fottea 10(1): 141–144, 2010 141 First report of Makinoella tosaensis OKADA (Chlorophyta, Chlorococcales, Oocystaceae) outside East Asia František HINDÁK & Alica HINDÁKOVÁ Institute of Botany, Slovak Academy of Sciences, Dúbravská cesta 9, SK–84523 Bratislava, Slovakia; e–mail: [email protected], [email protected] Abstract: The morphology of Makinoella tosaensis Okada 1949, a chlorococcalean coenobial alga described from Japan and since then observed only in Korea, has been studied under the LM from field material and in laboratory cultures. Its collection from a small fountain in the campus park of the Slovak Academy of Sciences in Bratislava, Slovakia, is the first record outside East Asia. The morphology of coenobia and cells in this material is in agreement with published data of the species. Key words: Chlorococcales, coenobial green algae, morphology, taxonomy, Slovakia Introduction & FOTT 1983). Outside of Japan (cf. http:// protist.i.hosei.ac.jp/PDB/Images/chlorophyta/ In the algal flora of Slovakia project, special Makinoella/index.html), it was observed only in attention was paid to small artificial water bodies Korea (he g e w a l d et al. 1999). The strain SAG such as urban fountains. The diversity of algae 28.97, isolated by Hegewald from Korea, is kept was investigated in several fountains within the at the Algal Collection in Göttingen, Germany. city limits of Bratislava (HINDÁK 1977, 1980 a, b, The ultrastructure of this culture was investigated 1984, 1988, 1990, 1996; HINDÁK & HINDÁKOVÁ in detail by sc h n e p f & he g e w a l d (2000). This 1994, 1998; HINDÁKOVÁ & HINDÁK 1998), strain was also used for molecular analyses and including a small basin located at the campus compared with some other representatives of the of the Slovak Academy of Sciences (SAS) at Oocystaceae (he p p e r l e et al. -

C O N F E R E N C E 12 6 January 2016
Joint Pathology Center Veterinary Pathology Services WEDNESDAY SLIDE CONFERENCE 2015-2016 C o n f e r e n c e 12 6 January 2016 Moderator: Tim Cooper, DVM, Ph.D, DACVP Associate Professor Department of Comparative Pathology Penn State Hershey Medical Center Hershey, PA CASE I: A10-9691 (JPC 3164430). Numerous fluid-filled cysts up to 7-8 mm in diameter were evident on cross-section. Signalment: Adult female guinea pig (Cavia Extensive stromal calcification or porcellus). ossification necessitated decalcification before histologic processing. History: This underweight adult guinea pig was found abandoned with truncal alopecia Laboratory Results: and a palpable abdominal mass. Age was No ancillary testing performed. estimated at 3 years. Ovariohysterectomy was performed in preparation for adoption. Histopathologic Description: A few viable follicles are in the periphery of the mass, but most of the specimen lacks recognizable ovarian tissue and instead is composed of neoplastic tissue from all 3 germ layers. The endodermal component includes tubuloacinar glands (lobules of serous acini formed by pyramidal cells with bright eosinophilic cytoplasmic granules) and cystic Ovary, guinea pig. The right ovary was enlarged at 3.5 cm x spaces lined by respiratory type epithelium 4 cm x 3.5 cm. (Photo courtesy of: Purdue University Animal Disease Diagnostic Laboratory: with numerous ciliated cells and mucus-filled http://www.addl.purdue.edu/ and Department of goblet cells. The ectodermal component Comparative Pathobiology: http://www.vet.purdue.) consists of neuroectodermal tissue with axons, glial cells, and neuronal cell bodies; Gross Pathology: The right ovary was no skin, hair follicles or cutaneous adnexal enlarged at 3.5 cm x 4 cm x 3.5 cm.