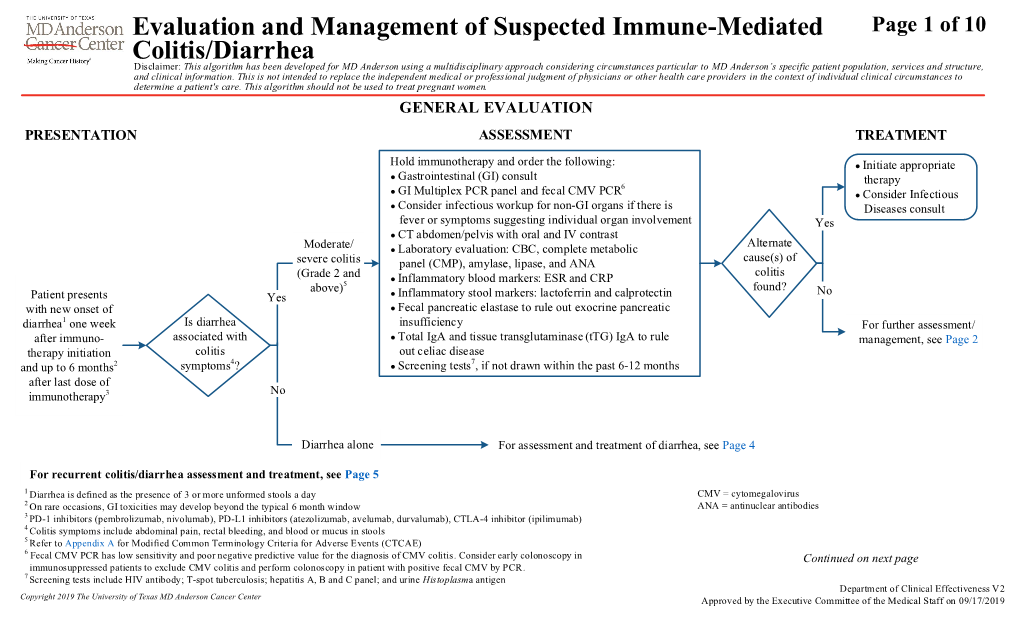
Evaluation and Management of Suspected Immune-Mediated
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genmab and ADC Therapeutics Announce Amended Agreement for Camidanlumab Tesirine (Cami)
Genmab and ADC Therapeutics Announce Amended Agreement for Camidanlumab Tesirine (Cami) Media Release Copenhagen, Denmark and Lausanne, Switzerland, October 30, 2020 • ADC Therapeutics to continue the development and commercialization of Cami • Genmab to receive mid-to-high single-digit tiered royalty Genmab A/S (Nasdaq: GMAB) and ADC Therapeutics SA (NYSE: ADCT) today announced that they have executed an amended agreement for ADC Therapeutics to continue the development and commercialization of camidanlumab tesirine (Cami). The parties first entered into a collaboration and license agreement in June 2013 for the development of Cami, an antibody drug conjugate (ADC) which combines Genmab’s HuMax®-TAC antibody targeting CD25 with ADC Therapeutics’ highly potent pyrrolobenzodiazepine (PBD) warhead technology. Under the terms of the 2013 agreement, the parties were to determine the path forward for continued development and commercialization of Cami upon completion of a Phase 1a/b clinical trial. ADC Therapeutics previously announced that Cami achieved an overall response rate of 86.5%, including a complete response rate of 48.6%, in Hodgkin lymphoma patients in this trial who had received a median of five prior lines of therapy. Cami is currently being evaluated in a 100-patient pivotal Phase 2 clinical trial intended to support the submission of a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA). The trial is more than 50 percent enrolled and ADC Therapeutics anticipates reporting interim results in the first half of 2021. “We have a long-standing relationship with the ADC Therapeutics team and believe they are an ideal partner for the ongoing development and potential commercialization of Cami,” said Jan van de Winkel, Ph.D., Chief Executive Officer of Genmab. -

ADC Therapeutics SA (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 6-K REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 UNDER THE SECURITIES EXCHANGE ACT OF 1934 For the month of February 2021. Commission File Number: 001-39071 ADC Therapeutics SA (Exact name of registrant as specified in its charter) Biopôle Route de la Corniche 3B 1066 Epalinges Switzerland (Address of principal executive office) Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F: Form 20-F ☒ Form 40-F Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐ Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐ SIGNATURE Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized. ADC Therapeutics SA Date: February 4, 2021 By: /s/ Michael Forer Name: Michael Forer Title: Executive Vice President & General Counsel EXHIBIT INDEX Exhibit No. Description 99.1 Press release dated February 4, 2021 Exhibit 99.1 ADC Therapeutics Completes Enrollment in Pivotal Phase 2 Clinical Trial of Camidanlumab Tesirine (Cami) in Relapsed or Refractory Hodgkin Lymphoma Interim results anticipated in the first half of 2021 LAUSANNE, Switzerland, February 4, 2021 – ADC Therapeutics SA (NYSE:ADCT), a late clinical-stage oncology-focused biotechnology company pioneering the development and commercialization of highly potent and targeted antibody drug conjugates (ADCs) for patients with hematological malignancies and solid tumors, today announced completion of enrollment in the pivotal Phase 2 clinical trial evaluating the efficacy and safety of camidanlumab tesirine (Cami, formerly ADCT-301) in patients with relapsed or refractory Hodgkin lymphoma. -

Positioning Biologics in the Management of Moderate to Severe Crohn's Disease
REVIEW CURRENT OPINION Positioning biologics in the management of moderate to severe Crohn’s disease Jana G. Hashash and Fadi H. Mourad Purpose of review Since there is a lack of head-to-head randomized controlled trials, little direction is provided from guidelines on the positioning of biologics for the treatment of Crohn’s disease (CD). This review utilizes comparative effectiveness and safety results from real-world data and network meta-analyses to inform clinical practice for positioning of biological therapies in the treatment of moderate-to-severe CD. Recent findings We summarize the results of studies pertaining to the identification of predictors for response to biologics in CD. Recently published studies about the management of moderate-to-severe CD are discussed and a positioning algorithm is proposed for the therapeutic approach of these patients. Summary Different classes of biologics are comparable with regards to safety and almost similar in effectiveness in the management of CD. There are certain clinical scenarios in which one biologic is more effective than another. For instance, patients with a more aggressive disease phenotype such as fistulizing disease would benefit from infliximab over other biologics, whereas in older patients at a higher risk for infectious complications, it may be more appropriate to use ustekinumab or vedolizumab over the anti-tumor necrosis factor (TNF) agents. More data pertaining to identifying predictors of response to the different available therapies and head-to-head comparison trials are needed to personalize our therapeutic approach of CD patients. Keywords biologics, Crohn’s disease, moderate-to-severe, positioning INTRODUCTION The medical management of patients with CD To guide the choice of treatment in patients with has changed over recent years. -

Inflammatory Bowel Disease Irritable Bowel Syndrome
Inflammatory Bowel Disease and Irritable Bowel Syndrome Similarities and Differences 2 www.ccfa.org IBD Help Center: 888.MY.GUT.PAIN 888.694.8872 Important Differences Between IBD and IBS Many diseases and conditions can affect the gastrointestinal (GI) tract, which is part of the digestive system and includes the esophagus, stomach, small intestine and large intestine. These diseases and conditions include inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS). IBD Help Center: 888.MY.GUT.PAIN 888.694.8872 www.ccfa.org 3 Inflammatory bowel diseases are a group of inflammatory conditions in which the body’s own immune system attacks parts of the digestive system. Inflammatory Bowel Disease Inflammatory bowel diseases are a group of inflamma- Causes tory conditions in which the body’s own immune system attacks parts of the digestive system. The two most com- The exact cause of IBD remains unknown. Researchers mon inflammatory bowel diseases are Crohn’s disease believe that a combination of four factors lead to IBD: a (CD) and ulcerative colitis (UC). IBD affects as many as 1.4 genetic component, an environmental trigger, an imbal- million Americans, most of whom are diagnosed before ance of intestinal bacteria and an inappropriate reaction age 35. There is no cure for IBD but there are treatments to from the immune system. Immune cells normally protect reduce and control the symptoms of the disease. the body from infection, but in people with IBD, the immune system mistakes harmless substances in the CD and UC cause chronic inflammation of the GI tract. CD intestine for foreign substances and launches an attack, can affect any part of the GI tract, but frequently affects the resulting in inflammation. -

ADC Therapeutics SA (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 6-K REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 UNDER THE SECURITIES EXCHANGE ACT OF 1934 For the month of July, 2020. Commission File Number: 001-39071 ADC Therapeutics SA (Exact name of registrant as specified in its charter) Biopôle Route de la Corniche 3B 1066 Epalinges Switzerland (Address of principal executive office) Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F: Form 20-F ☒ Form 40-F Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐ Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐ SIGNATURE Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized. ADC Therapeutics SA Date: July 6, 2020 By: /s/ Dominique Graz Name: Dominique Graz Title: General Counsel EXHIBIT INDEX Exhibit No. Description 99.1 Press release dated July 6, 2020 Exhibit 99.1 ADC Therapeutics Announces U.S. Food and Drug Administration Has Lifted Partial Clinical Hold on Pivotal Phase 2 Clinical Trial of Camidanlumab Tesirine Lausanne, Switzerland — July 6, 2020 — ADC Therapeutics SA (NYSE:ADCT), a clinical-stage oncology-focused biotechnology company leading the development and commercialization of next-generation antibody drug conjugates (ADCs) with highly potent and targeted pyrrolobenzodiazepine (PBD) dimer technology, today announced that the U.S. -

Comparative Effectiveness of Pembrolizumab Vs. Nivolumab In
www.nature.com/scientificreports OPEN Comparative efectiveness of pembrolizumab vs. nivolumab in patients with recurrent or advanced NSCLC Pengfei Cui1,2,4, Ruixin Li2,4, Ziwei Huang3,2,4, Zhaozhen Wu3,2, Haitao Tao2, Sujie Zhang2 & Yi Hu1,2,3* The efcacies of pembrolizumab and nivolumab have never been directly compared in a real-world study. Therefore, we sought to retrospectively evaluate the objective response rate (ORR) and the progression-free survival (PFS) of patients with recurrent or advanced non-small cell lung cancer (NSCLC) in a real-world setting. This study included patients with recurrent or advanced NSCLC diagnosed between September 1, 2015 and August 31, 2019, who were treated with programmed cell death 1 (PD-1) inhibitors at the Cancer Center of the Chinese People’s Liberation Army. PFS was estimated for each treatment group using Kaplan–Meier curves and log-rank tests. The multivariate analysis of PFS was performed with Cox proportional hazards regression models. A total of 255 patients with advanced or recurrent NSCLC treated with PD-1 inhibitors were identifed. The ORR was signifcantly higher in the pembrolizumab group than in the nivolumab group, while PFS was not signifcantly diferent between the two groups. Subgroup analysis showed that the ORR was signifcantly higher for pembrolizumab than for nivolumab in patients in the frst-line therapy subgroup and in those in the combination therapy as frst-line therapy subgroup. Survival analysis of patients receiving combination therapy as second- or further-line therapy showed that nivolumab had better efcacy than pembrolizumab. However, the multivariate analysis revealed no signifcant diference in PFS between patients treated with pembrolizumab and those treated with nivolumab regardless of the subgroup. -

Cutaneous Adverse Effects of Biologic Medications
REVIEW CME MOC Selena R. Pasadyn, BA Daniel Knabel, MD Anthony P. Fernandez, MD, PhD Christine B. Warren, MD, MS Cleveland Clinic Lerner College Department of Pathology Co-Medical Director of Continuing Medical Education; Department of Dermatology, Cleveland Clinic; of Medicine of Case Western and Department of Dermatology, W.D. Steck Chair of Clinical Dermatology; Director of Clinical Assistant Professor, Cleveland Clinic Reserve University, Cleveland, OH Cleveland Clinic Medical and Inpatient Dermatology; Departments of Lerner College of Medicine of Case Western Dermatology and Pathology, Cleveland Clinic; Assistant Reserve University, Cleveland, OH Clinical Professor, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH Cutaneous adverse effects of biologic medications ABSTRACT iologic therapy encompasses an expo- B nentially expanding arena of medicine. Biologic therapies have become widely used but often As the name implies, biologic therapies are de- cause cutaneous adverse effects. The authors discuss the rived from living organisms and consist largely cutaneous adverse effects of tumor necrosis factor (TNF) of proteins, sugars, and nucleic acids. A clas- alpha inhibitors, epidermal growth factor receptor (EGFR) sic example of an early biologic medication is inhibitors, small-molecule tyrosine kinase inhibitors insulin. These therapies have revolutionized (TKIs), and cell surface-targeted monoclonal antibodies, medicine and offer targeted therapy for an including how to manage these reactions -

Chelsea & Westminster Healthcare Trust Drugs Committee
Chelsea and Westminster Hospital NHS Foundation Trust Trust Medicines Group Summary of Main Points from the Meeting held on Monday 12th December 2016 2. Minutes and Summary Notes from last meeting The Minutes and Summary notes from the November 2016 Medicines Group meeting were approved and will be circulated. 3. Matters Arising The Group noted the matters arising from the previous meeting. 4. Business to be transacted by the Medicines Group 4.1 Formulary Applications Ex-panel Deferasirox (Exjade®) 90mg, 180mg and 360mg Tablets (Novartis) Exjade® film coated tablets have been introduced in November 2016. These will take the place of the dispersible tabs which will be phased out and no longer available from June 2017. Costs are equivalent to the dispersible tablets. The new film coated tablets have a number of advantages over the dispersible tablets including: - Can be swallowed whole - Lactose free - No food restrictions - No mixing/preparation required. Decision: Approved DuoResp Spiromax® 160 micrograms / 4.5 micrograms (Budesonide & Formoterol) Inhalation Powder (Teva) Request by the Respiratory Team for the management of asthma in line with the BTS guidelines where a ® combination of an inhaled long acting B2 agonist and corticosteroid is indicated. Adding DuoResp Spiromax to the formulary will provide an increased choice of devices available to prescribe. Symbicort Turbohaler® is already included on the formulary. DuoResp Spiromax® is more cost-effective than Symbicort Turbohaler® and is currently included on the NWLIF and GPs have already started to prescribe it. Decision: Approved Sodium Valpoate (Epilim Chronosphere®) 50mg and 100mg Modified Release Granules (Sanofi) Requested by the Paediatric Neurology Team for paediatric patients requiring sodium valpoate in very low doses. -

B.C. Pharmacare Drug Information Sheet for Vedolizumab (Entyvio)
The drug below is being considered for possible coverage under the B.C. PharmaCare program. PharmaCare is a government-funded drug plan that helps British Columbians with the cost of eligible prescription drugs and specific medical supplies. For more information on PharmaCare, visit Ministry of Health - PharmaCare. PharmaCare reviews each drug for treating a specific illness or medical condition (known as an “indication”). If a decision is made to cover the drug, it will be only for that illness or condition. In some cases, PharmaCare may cover a drug only for people who have the illness or condition and have not responded to other drugs used to treat that illness or condition. For more information on PharmaCare’s drug coverage review process, see the last page of this information sheet. Information about the drug Generic name (scientific name) vedolizumab Brand name Entyvio® Manufacturer Takeda Canada Inc. Indication Entyvio is indicated for the treatment of moderately to severely active Crohn’s disease in adult patients. Has the drug been reviewed by Yes the Common Drug For more information about the CDR’s review of vedolizumab (Entyvio®), you can Review (CDR)? Search the CDR Reports. (see the note below this table.) Public input start date Friday, November 13, 2020 Public input closing date Friday, December 11, 2020 AT MIDNIGHT How is the drug taken? Entyvio is administered by subcutaneous injection (into the skin). How often is the drug taken? Entyvio is administered every two weeks, following at least two intravenous infusions (into the vein). Ministry of Health Pharmaceutical Services Division Page 1 of 4 BC PharmaCare Drug Information — vedolizumab (Entyvio®) continued… Information about the drug General drug and/or drug Crohn’s disease is a chronic inflammatory disease of the gastrointestinal tract, which study information most commonly affects the small intestine, beginning of large intestine, and rectum. -

Risk Assessment and Risk Mitigation Review(S)
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 125476Orig1s000 RISK ASSESSMENT and RISK MITIGATION REVIEW(S) Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research Office of Surveillance and Epidemiology Office of Medication Error Prevention and Risk Management Final Risk Evaluation and Mitigation Strategy (REMS) Review Date: May 7, 2014 Reviewer(s): George Neyarapally, Pharm.D., M.P.H. Division of Risk Management (DRISK) Team Leader: Reema Mehta, Pharm.D., M.P.H. DRISK Division Director: Claudia Manzo, Pharm.D. DRISK Drug Name(s): Entyvio (vedolizumab) Therapeutic Class: Lymphocyte α4β7 integrin receptor antagonist Dosage and Route: Solution for infusion Application Type/Number: BLA 125476, BLA 125507 Applicant/Sponsor: Takeda Pharmaceuticals OSE RCM #: 2013-1641 *** This document contains proprietary and confidential information that should not be released to the public. *** 1 Reference ID: 3502774 CONTENTS 1 INTRODUCTION ....................................................................................................... 1 1.1 Product Background............................................................................................ 1 1.2 Disease Background............................................................................................ 2 1.3 Regulatory History .............................................................................................. 2 2 MATERIALS REVIEWED ....................................................................................... -

Chronic Viral Hepatitis in a Cohort of Inflammatory Bowel Disease
pathogens Article Chronic Viral Hepatitis in a Cohort of Inflammatory Bowel Disease Patients from Southern Italy: A Case-Control Study Giuseppe Losurdo 1,2 , Andrea Iannone 1, Antonella Contaldo 1, Michele Barone 1 , Enzo Ierardi 1 , Alfredo Di Leo 1,* and Mariabeatrice Principi 1 1 Section of Gastroenterology, Department of Emergency and Organ Transplantation, University “Aldo Moro” of Bari, 70124 Bari, Italy; [email protected] (G.L.); [email protected] (A.I.); [email protected] (A.C.); [email protected] (M.B.); [email protected] (E.I.); [email protected] (M.P.) 2 Ph.D. Course in Organs and Tissues Transplantation and Cellular Therapies, Department of Emergency and Organ Transplantation, University “Aldo Moro” of Bari, 70124 Bari, Italy * Correspondence: [email protected]; Tel.: +39-080-559-2925 Received: 14 September 2020; Accepted: 21 October 2020; Published: 23 October 2020 Abstract: We performed an epidemiologic study to assess the prevalence of chronic viral hepatitis in inflammatory bowel disease (IBD) and to detect their possible relationships. Methods: It was a single centre cohort cross-sectional study, during October 2016 and October 2017. Consecutive IBD adult patients and a control group of non-IBD subjects were recruited. All patients underwent laboratory investigations to detect chronic hepatitis B (HBV) and C (HCV) infection. Parameters of liver function, elastography and IBD features were collected. Univariate analysis was performed by Student’s t or chi-square test. Multivariate analysis was performed by binomial logistic regression and odds ratios (ORs) were calculated. We enrolled 807 IBD patients and 189 controls. Thirty-five (4.3%) had chronic viral hepatitis: 28 HCV (3.4%, versus 5.3% in controls, p = 0.24) and 7 HBV (0.9% versus 0.5% in controls, p = 0.64). -

Ulcerative Colitis: Diagnosis and Treatment ROBERT C
Ulcerative Colitis: Diagnosis and Treatment ROBERT C. LANGAN, MD; PATRICIA B. GOTSCH, MD; MICHAEL A. KRAFCZYK, MD; and DAVID D. SKILLINGE, DO, St. Luke’s Family Medicine Residency, Bethlehem, Pennsylvania Ulcerative colitis is a chronic disease with recurrent symptoms and significant morbidity. The precise etiology is still unknown. As many as 25 percent of patients with ulcerative colitis have extraintestinal manifestations. The diagnosis is made endoscopically. Tests such as perinuclear antineutrophilic cytoplasmic antibodies and anti-Saccharomyces cerevisiae antibodies are promising, but not yet recommended for routine use. Treatment is based on the extent and severity of the disease. Rectal therapy with 5-aminosalicylic acid compounds is used for proc- titis. More extensive disease requires treatment with oral 5-aminosalicylic acid compounds and oral corticosteroids. The side effects of steroids limit their usefulness for chronic therapy. Patients who do not respond to treatment with oral corticosteroids require hospitalization and intravenous steroids. Refractory symptoms may be treated with azathioprine or infliximab. Surgical treatment of ulcerative colitis is reserved for patients who fail medical therapy or who develop severe hemorrhage, perforation, or cancer. Longstanding ulcerative colitis is associated with an increased risk of colon cancer. Patients should receive an initial screening colonos- copy eight years after the onset of pancolitis and 12 to 15 years after the onset of left-sided dis- ease; follow-up colonoscopy should be repeated every two to three years. (Am Fam Physician 2007;76:1323-30, 1331. Copyright © 2007 American Academy of Family Physicians.) This article exempli- lcerative colitis is a chronic dis- of ulcerative colitis is not well understood.