Developmental Neuropathology
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
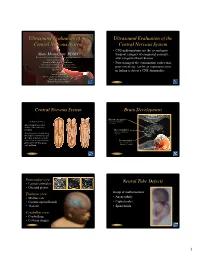
Ultrasound Evaluation of the Central Nervous System
Ultrasound Evaluation of the Ultrasound Evaluation of the Central Nervous System Central Nervous System ••CNSCNS malformations are the second most Mani Montazemi, RDMS frequent category of congenital anomaly, Director of Ultrasound Education & Quality Assurancee after congenital heart disease Baylor College of Medicine Division of Maternal-Fetal Medicine ••PoorPoor timing of the examination, rather than Department of Obstetrics and Gynecology Texas Children’s Hospital, Pavilion for Women poor sensitivity, can be an important factor Houston Texas & in failing to detect a CNS abnormality Clinical Instructor Thomas Jefferson University Hospital Radiology Department Fetal Head Philadelphia, Pennsylvania Fetal Head Central Nervous System Brain Development 9 -13 weeks Rhombencephalon 5th Menstrual Week •Gives rise to hindbrain •4th ventricle Arises from the posterior surface of the embryonic ectoderm Mesencephalon •Gives rise to midbrain A small groove is found along •Aqueduct the midline of the embryo and the edges of this groove fold over to form a neuro tube that Prosencephalon gives rise to the fetal spinal •Gives rise to forebrain rd cord and brain •Lateral & 3 ventricles Fetal Head Fetal Head Ventricular view Neural Tube Defects ••LateralLateral ventricles ••ChoroidChoroid plexus Group of malformations: Thalamic view • Anencephaly ••MidlineMidline falx •Anencephaly ••CavumCavum septiseptipellucidi pellucidi ••CephalocelesCephaloceles ••ThalamiThalami ••SpinaSpina bifida Cerebellar view ••CerebellumCerebellum ••CisternaCisterna magna Fetal -

Approach to Brain Malformations
Approach to Brain Malformations A General Imaging Approach to Brain CSF spaces. This is the basis for development of the Dandy- Malformations Walker malformation; it requires abnormal development of the cerebellum itself and of the overlying leptomeninges. Whenever an infant or child is referred for imaging because of Looking at the midline image also gives an idea of the relative either seizures or delayed development, the possibility of a head size through assessment of the craniofacial ratio. In the brain malformation should be carefully investigated. If the normal neonate, the ratio of the cranial vault to the face on child appears dysmorphic in any way (low-set ears, abnormal midline images is 5:1 or 6:1. By 2 years, it should be 2.5:1, and facies, hypotelorism), the likelihood of an underlying brain by 10 years, it should be about 1.5:1. malformation is even higher, but a normal appearance is no guarantee of a normal brain. In all such cases, imaging should After looking at the midline, evaluate the brain from outside be geared toward showing a structural abnormality. The to inside. Start with the cerebral cortex. Is the thickness imaging sequences should maximize contrast between gray normal (2-3 mm)? If it is too thick, think of pachygyria or matter and white matter, have high spatial resolution, and be polymicrogyria. Is the cortical white matter junction smooth or acquired as volumetric data whenever possible so that images irregular? If it is irregular, think of polymicrogyria or Brain: Pathology-Based Diagnoses can be reformatted in any plane or as a surface rendering. -

A Anencephaly
Glossary of Birth Anomaly Terms: A Anencephaly: A deadly birth anomaly where most of the brain and skull did not form. Anomaly: Any part of the body or chromosomes that has an unusual or irregular structure. Aortic valve stenosis: The aortic valve controls the flow of blood from the left ventricle of the heart to the aorta, which takes the blood to the rest of the body. If there is stenosis of this valve, the valve has space for blood to flow through, but it is too narrow. Atresia: Lack of an opening where there should be one. Atrial septal defect: An opening in the wall (septum) that separates the left and right top chambers (atria) of the heart. A hole can vary in size and may close on its own or may require surgery. Atrioventricular septal defect (endocardial cushion defect): A defect in both the lower portion of the atrial septum and the upper portion of the ventricular septum. Together, these defects make a large opening (canal) in the middle part of the heart. Aniridia (an-i-rid-e-a): An eye anomaly where the colored part of the eye (called the iris) is partly or totally missing. It usually affects both eyes. Other parts of the eye can also be formed incorrectly. The effects on children’s ability to see can range from mild problems to blindness. To learn more about aniridia, go to the U.S. National Library of Medicine website. Anophthalmia/microphthalmia (an-oph-thal-mia/mi-croph-thal-mia): Birth anomalies of the eyes. In anophthalmia, a baby is born without one or both eyes. -

Congenital Disorders of Glycosylation from a Neurological Perspective
brain sciences Review Congenital Disorders of Glycosylation from a Neurological Perspective Justyna Paprocka 1,* , Aleksandra Jezela-Stanek 2 , Anna Tylki-Szyma´nska 3 and Stephanie Grunewald 4 1 Department of Pediatric Neurology, Faculty of Medical Science in Katowice, Medical University of Silesia, 40-752 Katowice, Poland 2 Department of Genetics and Clinical Immunology, National Institute of Tuberculosis and Lung Diseases, 01-138 Warsaw, Poland; [email protected] 3 Department of Pediatrics, Nutrition and Metabolic Diseases, The Children’s Memorial Health Institute, W 04-730 Warsaw, Poland; [email protected] 4 NIHR Biomedical Research Center (BRC), Metabolic Unit, Great Ormond Street Hospital and Institute of Child Health, University College London, London SE1 9RT, UK; [email protected] * Correspondence: [email protected]; Tel.: +48-606-415-888 Abstract: Most plasma proteins, cell membrane proteins and other proteins are glycoproteins with sugar chains attached to the polypeptide-glycans. Glycosylation is the main element of the post- translational transformation of most human proteins. Since glycosylation processes are necessary for many different biological processes, patients present a diverse spectrum of phenotypes and severity of symptoms. The most frequently observed neurological symptoms in congenital disorders of glycosylation (CDG) are: epilepsy, intellectual disability, myopathies, neuropathies and stroke-like episodes. Epilepsy is seen in many CDG subtypes and particularly present in the case of mutations -

Pushing the Limits of Prenatal Ultrasound: a Case of Dorsal Dermal Sinus Associated with an Overt Arnold–Chiari Malformation and a 3Q Duplication
reproductive medicine Case Report Pushing the Limits of Prenatal Ultrasound: A Case of Dorsal Dermal Sinus Associated with an Overt Arnold–Chiari Malformation and a 3q Duplication Olivier Leroij 1, Lennart Van der Veeken 2,*, Bettina Blaumeiser 3 and Katrien Janssens 3 1 Faculty of Medicine, University of Antwerp, 2610 Wilrijk, Belgium; [email protected] 2 Department of Obstetrics and Gynaecology, University Hospital Antwerp, 2650 Edegem, Belgium 3 Department of Medical Genetics, University Hospital and University of Antwerp, 2650 Edegem, Belgium; [email protected] (B.B.); [email protected] (K.J.) * Correspondence: [email protected] Abstract: We present a case of a fetus with cranial abnormalities typical of open spina bifida but with an intact spine shown on both ultrasound and fetal MRI. Expert ultrasound examination revealed a very small tract between the spine and the skin, and a postmortem examination confirmed the diagnosis of a dorsal dermal sinus. Genetic analysis found a mosaic 3q23q27 duplication in the form of a marker chromosome. This case emphasizes that meticulous prenatal ultrasound examination has the potential to diagnose even closed subtypes of neural tube defects. Furthermore, with cerebral anomalies suggesting a spina bifida, other imaging techniques together with genetic tests and measurement of alpha-fetoprotein in the amniotic fluid should be performed. Citation: Leroij, O.; Van der Veeken, Keywords: dorsal dermal sinus; Arnold–Chiari anomaly; 3q23q27 duplication; mosaic; marker chro- L.; Blaumeiser, B.; Janssens, K. mosome Pushing the Limits of Prenatal Ultrasound: A Case of Dorsal Dermal Sinus Associated with an Overt Arnold–Chiari Malformation and a 3q 1. -

Polymicrogyria (PMG) ‘Many–Small–Folds’
Polymicrogyria Dr Andrew Fry Clinical Senior Lecturer in Medical Genetics Institute of Medical Genetics, Cardiff [email protected] Polymicrogyria (PMG) ‘Many–small–folds’ • PMG is heterogeneous – in aetiology and phenotype • A disorder of post-migrational cortical organisation. PMG often appears thick on MRI with blurring of the grey-white matter boundary Normal PMG On MRI PMG looks thick but the cortex is actually thin – with folded, fused gyri Courtesy of Dr Jeff Golden, Pen State Unv, Philadelphia PMG is often confused with pachygyria (lissencephaly) Thick cortex (10 – 20mm) Axial MRI 4 cortical layers Lissencephaly Polymicrogyria Cerebrum Classical lissencephaly is due Many small gyri – often to under-migration. fused together. Axial MRI image at 7T showing morphological aspects of PMG. Guerrini & Dobyns Malformations of cortical development: clinical features and genetic causes. Lancet Neurol. 2014 Jul; 13(7): 710–726. PMG - aetiology Pregnancy history • Intrauterine hypoxic/ischemic brain injury (e.g. death of twin) • Intrauterine infection (e.g. CMV, Zika virus) TORCH, CMV PCR, [+deafness & cerebral calcification] CT scan • Metabolic (e.g. Zellweger syndrome, glycine encephalopathy) VLCFA, metabolic Ix • Genetic: Family history Familial recurrence (XL, AD, AR) Chromosomal abnormalities (e.g. 1p36 del, 22q11.2 del) Syndromic (e.g. Aicardi syndrome, Kabuki syndrome) Examin - Monogenic (e.g. TUBB2B, TUBA1A, GPR56) Array ation CGH Gene test/Panel/WES/WGS A cohort of 121 PMG patients Aim: To explore the natural history of PMG and identify new genes. Recruited: • 99 unrelated patients • 22 patients from 10 families 87% White British, 53% male ~92% sporadic cases (NB. ascertainment bias) Sporadic PMG • Array CGH, single gene and gene panel testing - then a subset (n=57) had trio-WES. -

Massachusetts Birth Defects 2002-2003
Massachusetts Birth Defects 2002-2003 Massachusetts Birth Defects Monitoring Program Bureau of Family Health and Nutrition Massachusetts Department of Public Health January 2008 Massachusetts Birth Defects 2002-2003 Deval L. Patrick, Governor Timothy P. Murray, Lieutenant Governor JudyAnn Bigby, MD, Secretary, Executive Office of Health and Human Services John Auerbach, Commissioner, Massachusetts Department of Public Health Sally Fogerty, Director, Bureau of Family Health and Nutrition Marlene Anderka, Director, Massachusetts Center for Birth Defects Research and Prevention Linda Casey, Administrative Director, Massachusetts Center for Birth Defects Research and Prevention Cathleen Higgins, Birth Defects Surveillance Coordinator Massachusetts Department of Public Health 617-624-5510 January 2008 Acknowledgements This report was prepared by the staff of the Massachusetts Center for Birth Defects Research and Prevention (MCBDRP) including: Marlene Anderka, Linda Baptiste, Elizabeth Bingay, Joe Burgio, Linda Casey, Xiangmei Gu, Cathleen Higgins, Angela Lin, Rebecca Lovering, and Na Wang. Data in this report have been collected through the efforts of the field staff of the MCBDRP including: Roberta Aucoin, Dorothy Cichonski, Daniel Sexton, Marie-Noel Westgate and Susan Winship. We would like to acknowledge the following individuals for their time and commitment to supporting our efforts in improving the MCBDRP. Lewis Holmes, MD, Massachusetts General Hospital Carol Louik, ScD, Slone Epidemiology Center, Boston University Allen Mitchell, -

CONGENITAL ABNORMALITIES of the CENTRAL NERVOUS SYSTEM Christopher Verity, Helen Firth, Charles Ffrench-Constant *I3
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.74.suppl_1.i3 on 1 March 2003. Downloaded from CONGENITAL ABNORMALITIES OF THE CENTRAL NERVOUS SYSTEM Christopher Verity, Helen Firth, Charles ffrench-Constant *i3 J Neurol Neurosurg Psychiatry 2003;74(Suppl I):i3–i8 dvances in genetics and molecular biology have led to a better understanding of the control of central nervous system (CNS) development. It is possible to classify CNS abnormalities Aaccording to the developmental stages at which they occur, as is shown below. The careful assessment of patients with these abnormalities is important in order to provide an accurate prog- nosis and genetic counselling. c NORMAL DEVELOPMENT OF THE CNS Before we review the various abnormalities that can affect the CNS, a brief overview of the normal development of the CNS is appropriate. c Induction—After development of the three cell layers of the early embryo (ectoderm, mesoderm, and endoderm), the underlying mesoderm (the “inducer”) sends signals to a region of the ecto- derm (the “induced tissue”), instructing it to develop into neural tissue. c Neural tube formation—The neural ectoderm folds to form a tube, which runs for most of the length of the embryo. c Regionalisation and specification—Specification of different regions and individual cells within the neural tube occurs in both the rostral/caudal and dorsal/ventral axis. The three basic regions of copyright. the CNS (forebrain, midbrain, and hindbrain) develop at the rostral end of the tube, with the spinal cord more caudally. Within the developing spinal cord specification of the different popu- lations of neural precursors (neural crest, sensory neurones, interneurones, glial cells, and motor neurones) is observed in progressively more ventral locations. -

Chiari Type II Malformation: Past, Present, and Future
Neurosurg Focus 16 (2):Article 5, 2004, Click here to return to Table of Contents Chiari Type II malformation: past, present, and future KEVIN L. STEVENSON, M.D. Children’s Healthcare of Atlanta, Atlanta, Georgia Object. The Chiari Type II malformation (CM II) is a unique hindbrain herniation found only in patients with myelomeningocele and is the leading cause of death in these individuals younger than 2 years of age. Several theories exist as to its embryological evolution and recently new theories are emerging as to its treatment and possible preven- tion. A thorough understanding of the embryology, anatomy, symptomatology, and surgical treatment is necessary to care optimally for children with myelomeningocele and prevent significant morbidity and mortality. Methods. A review of the literature was used to summarize the clinically pertinent features of the CM II, with par- ticular attention to pitfalls in diagnosis and surgical treatment. Conclusions. Any child with CM II can present as a neurosurgical emergency. Expeditious and knowledgeable eval- uation and prompt surgical decompression of the hindbrain can prevent serious morbidity and mortality in the patient with myelomeningocele, especially those younger than 2 years old. Symptomatic CM II in the older child often pre- sents with more subtle findings but rarely in acute crisis. Understanding of CM II continues to change as innovative techniques are applied to this challenging patient population. KEY WORDS • Chiari Type II malformation • myelomeningocele • pediatric The CM II is uniquely associated with myelomeningo- four distinct forms of the malformation, including the cele and is found only in this population. Originally de- Type II malformation that he found exclusively in patients scribed by Hans Chiari in 1891, symptomatic CM II ac- with myelomeningocele. -

Auditory Processing Disorders in Twins with Perisylvian Polymicrogyria
Arq Neuropsiquiatr 2009;67(2-B):499-501 Clinical / Scientific note AUDITORY PROCESSING DISORDERS IN TWINS WITH PERISYLVIAN POLYMICROGYRIA Mirela Boscariol1, Vera Lúcia Garcia2, Catarina A. Guimarães3, Simone R.V. Hage4, Maria Augusta Montenegro5, Fernando Cendes6, Marilisa M. Guerreiro7 Bilateral perisylvian polymicrogyria is a malformation tigation was performed in a 2.0 T scanner (Elscint Prestige) with of cortical development due to abnormal late neuronal posterior multiplanar reconstruction and curvilinear reformat- migration or abnormal cortical organization around the ting in 3D magnetic resonance imaging (MRI). sylvian fissure1. The language assessment considered the following aspects: The severity of the clinical manifestations correlates phonological, morphosyntactic, semantic and pragmatic produc- with the extent of the lesion. Therefore, the term diffuse tion. Standard and non-standard speech protocols were used: polymicrogyria is applied when the cortical malforma- sample of free speech; ABFW – Children Language Test with tion spreads around the entire sylvian fissure, and restrict- phonological and vocabulary tests3. Reading/writing evaluation ed polymicrogyria is applied when polymicrogyria occurs included: sample of free writing, Phonologic Skill Test4, School only in the posterior part of the parietal region. The re- Performance Test5, non-words reading and writing, oral speed stricted form is also called bilateral posterior parietal reading, and text understanding. polymicrogyria and appears to be associated with a genet- The peripheral audiological capability was assessed with au- ic predisposition and soft clinical features (such as speech diometry, speech reception thresholds and acoustic impedance delay and dysarthria) when compared to the diffuse form tests. An acoustic cabin was used with an AC-30 audiometer (In- of polymicrogyria. -

Classification of Congenital Abnormalities of the CNS
315 Classification of Congenital Abnormalities of the CNS M. S. van der Knaap1 A classification of congenital cerebral, cerebellar, and spinal malformations is pre J . Valk2 sented with a view to its practical application in neuroradiology. The classification is based on the MR appearance of the morphologic abnormalities, arranged according to the embryologic time the derangement occurred. The normal embryology of the brain is briefly reviewed, and comments are made to explain the classification. MR images illustrating each subset of abnormalities are presented. During the last few years, MR imaging has proved to be a diagnostic tool of major importance in children with congenital malformations of the eNS [1]. The excellent gray fwhite-matter differentiation and multi planar imaging capabilities of MR allow a systematic analysis of the condition of the brain in infants and children. This is of interest for estimating prognosis and for genetic counseling. A classification is needed to serve as a guide to the great diversity of morphologic abnormalities and to make the acquired data useful. Such a system facilitates encoding, storage, and computer processing of data. We present a practical classification of congenital cerebral , cerebellar, and spinal malformations. Our classification is based on the morphologic abnormalities shown by MR and on the time at which the derangement of neural development occurred. A classification based on etiology is not as valuable because the various presumed causes rarely lead to a specific pattern of malformations. The abnor malities reflect the time the noxious agent interfered with neural development, rather than the nature of the noxious agent. The vulnerability of the various structures to adverse agents is greatest during the period of most active growth and development. -

Supratentorial Brain Malformations
Supratentorial Brain Malformations Edward Yang, MD PhD Department of Radiology Boston Children’s Hospital 1 May 2015/ SPR 2015 Disclosures: Consultant, Corticometrics LLC Objectives 1) Review major steps in the morphogenesis of the supratentorial brain. 2) Categorize patterns of malformation that result from failure in these steps. 3) Discuss particular imaging features that assist in recognition of these malformations. 4) Reference some of the genetic bases for these malformations to be discussed in greater detail later in the session. Overview I. Schematic overview of brain development II. Abnormalities of hemispheric cleavage III. Commissural (Callosal) abnormalities IV. Migrational abnormalities - Gray matter heterotopia - Pachygyria/Lissencephaly - Focal cortical dysplasia - Transpial migration - Polymicrogyria V. Global abnormalities in size (proliferation) VI. Fetal Life and Myelination Considerations I. Schematic Overview of Brain Development Embryology Top Mid-sagittal Top Mid-sagittal Closed Neural Tube (4 weeks) Corpus Callosum Callosum Formation Genu ! Splenium Cerebral Hemisphere (11-20 weeks) Hemispheric Cleavage (4-6 weeks) Neuronal Migration Ventricular/Subventricular Zones Ventricle ! Cortex (8-24 weeks) Neuronal Precursor Generation (Proliferation) (6-16 weeks) Embryology From ten Donkelaar Clinical Neuroembryology 2010 4mo 6mo 8mo term II. Abnormalities of Hemispheric Cleavage Holoprosencephaly (HPE) Top Mid-sagittal Imaging features: Incomplete hemispheric separation + 1)1) No septum pellucidum in any HPEs Closed Neural