Version 1.0, 8/21/2016 Zika Pregnancy Outcome Reporting Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
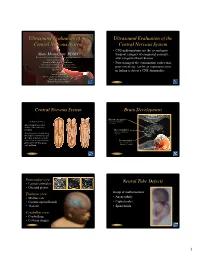
Ultrasound Evaluation of the Central Nervous System
Ultrasound Evaluation of the Ultrasound Evaluation of the Central Nervous System Central Nervous System ••CNSCNS malformations are the second most Mani Montazemi, RDMS frequent category of congenital anomaly, Director of Ultrasound Education & Quality Assurancee after congenital heart disease Baylor College of Medicine Division of Maternal-Fetal Medicine ••PoorPoor timing of the examination, rather than Department of Obstetrics and Gynecology Texas Children’s Hospital, Pavilion for Women poor sensitivity, can be an important factor Houston Texas & in failing to detect a CNS abnormality Clinical Instructor Thomas Jefferson University Hospital Radiology Department Fetal Head Philadelphia, Pennsylvania Fetal Head Central Nervous System Brain Development 9 -13 weeks Rhombencephalon 5th Menstrual Week •Gives rise to hindbrain •4th ventricle Arises from the posterior surface of the embryonic ectoderm Mesencephalon •Gives rise to midbrain A small groove is found along •Aqueduct the midline of the embryo and the edges of this groove fold over to form a neuro tube that Prosencephalon gives rise to the fetal spinal •Gives rise to forebrain rd cord and brain •Lateral & 3 ventricles Fetal Head Fetal Head Ventricular view Neural Tube Defects ••LateralLateral ventricles ••ChoroidChoroid plexus Group of malformations: Thalamic view • Anencephaly ••MidlineMidline falx •Anencephaly ••CavumCavum septiseptipellucidi pellucidi ••CephalocelesCephaloceles ••ThalamiThalami ••SpinaSpina bifida Cerebellar view ••CerebellumCerebellum ••CisternaCisterna magna Fetal -

A Anencephaly
Glossary of Birth Anomaly Terms: A Anencephaly: A deadly birth anomaly where most of the brain and skull did not form. Anomaly: Any part of the body or chromosomes that has an unusual or irregular structure. Aortic valve stenosis: The aortic valve controls the flow of blood from the left ventricle of the heart to the aorta, which takes the blood to the rest of the body. If there is stenosis of this valve, the valve has space for blood to flow through, but it is too narrow. Atresia: Lack of an opening where there should be one. Atrial septal defect: An opening in the wall (septum) that separates the left and right top chambers (atria) of the heart. A hole can vary in size and may close on its own or may require surgery. Atrioventricular septal defect (endocardial cushion defect): A defect in both the lower portion of the atrial septum and the upper portion of the ventricular septum. Together, these defects make a large opening (canal) in the middle part of the heart. Aniridia (an-i-rid-e-a): An eye anomaly where the colored part of the eye (called the iris) is partly or totally missing. It usually affects both eyes. Other parts of the eye can also be formed incorrectly. The effects on children’s ability to see can range from mild problems to blindness. To learn more about aniridia, go to the U.S. National Library of Medicine website. Anophthalmia/microphthalmia (an-oph-thal-mia/mi-croph-thal-mia): Birth anomalies of the eyes. In anophthalmia, a baby is born without one or both eyes. -

Pushing the Limits of Prenatal Ultrasound: a Case of Dorsal Dermal Sinus Associated with an Overt Arnold–Chiari Malformation and a 3Q Duplication
reproductive medicine Case Report Pushing the Limits of Prenatal Ultrasound: A Case of Dorsal Dermal Sinus Associated with an Overt Arnold–Chiari Malformation and a 3q Duplication Olivier Leroij 1, Lennart Van der Veeken 2,*, Bettina Blaumeiser 3 and Katrien Janssens 3 1 Faculty of Medicine, University of Antwerp, 2610 Wilrijk, Belgium; [email protected] 2 Department of Obstetrics and Gynaecology, University Hospital Antwerp, 2650 Edegem, Belgium 3 Department of Medical Genetics, University Hospital and University of Antwerp, 2650 Edegem, Belgium; [email protected] (B.B.); [email protected] (K.J.) * Correspondence: [email protected] Abstract: We present a case of a fetus with cranial abnormalities typical of open spina bifida but with an intact spine shown on both ultrasound and fetal MRI. Expert ultrasound examination revealed a very small tract between the spine and the skin, and a postmortem examination confirmed the diagnosis of a dorsal dermal sinus. Genetic analysis found a mosaic 3q23q27 duplication in the form of a marker chromosome. This case emphasizes that meticulous prenatal ultrasound examination has the potential to diagnose even closed subtypes of neural tube defects. Furthermore, with cerebral anomalies suggesting a spina bifida, other imaging techniques together with genetic tests and measurement of alpha-fetoprotein in the amniotic fluid should be performed. Citation: Leroij, O.; Van der Veeken, Keywords: dorsal dermal sinus; Arnold–Chiari anomaly; 3q23q27 duplication; mosaic; marker chro- L.; Blaumeiser, B.; Janssens, K. mosome Pushing the Limits of Prenatal Ultrasound: A Case of Dorsal Dermal Sinus Associated with an Overt Arnold–Chiari Malformation and a 3q 1. -

CONGENITAL ABNORMALITIES of the CENTRAL NERVOUS SYSTEM Christopher Verity, Helen Firth, Charles Ffrench-Constant *I3
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.74.suppl_1.i3 on 1 March 2003. Downloaded from CONGENITAL ABNORMALITIES OF THE CENTRAL NERVOUS SYSTEM Christopher Verity, Helen Firth, Charles ffrench-Constant *i3 J Neurol Neurosurg Psychiatry 2003;74(Suppl I):i3–i8 dvances in genetics and molecular biology have led to a better understanding of the control of central nervous system (CNS) development. It is possible to classify CNS abnormalities Aaccording to the developmental stages at which they occur, as is shown below. The careful assessment of patients with these abnormalities is important in order to provide an accurate prog- nosis and genetic counselling. c NORMAL DEVELOPMENT OF THE CNS Before we review the various abnormalities that can affect the CNS, a brief overview of the normal development of the CNS is appropriate. c Induction—After development of the three cell layers of the early embryo (ectoderm, mesoderm, and endoderm), the underlying mesoderm (the “inducer”) sends signals to a region of the ecto- derm (the “induced tissue”), instructing it to develop into neural tissue. c Neural tube formation—The neural ectoderm folds to form a tube, which runs for most of the length of the embryo. c Regionalisation and specification—Specification of different regions and individual cells within the neural tube occurs in both the rostral/caudal and dorsal/ventral axis. The three basic regions of copyright. the CNS (forebrain, midbrain, and hindbrain) develop at the rostral end of the tube, with the spinal cord more caudally. Within the developing spinal cord specification of the different popu- lations of neural precursors (neural crest, sensory neurones, interneurones, glial cells, and motor neurones) is observed in progressively more ventral locations. -

Classification of Congenital Abnormalities of the CNS
315 Classification of Congenital Abnormalities of the CNS M. S. van der Knaap1 A classification of congenital cerebral, cerebellar, and spinal malformations is pre J . Valk2 sented with a view to its practical application in neuroradiology. The classification is based on the MR appearance of the morphologic abnormalities, arranged according to the embryologic time the derangement occurred. The normal embryology of the brain is briefly reviewed, and comments are made to explain the classification. MR images illustrating each subset of abnormalities are presented. During the last few years, MR imaging has proved to be a diagnostic tool of major importance in children with congenital malformations of the eNS [1]. The excellent gray fwhite-matter differentiation and multi planar imaging capabilities of MR allow a systematic analysis of the condition of the brain in infants and children. This is of interest for estimating prognosis and for genetic counseling. A classification is needed to serve as a guide to the great diversity of morphologic abnormalities and to make the acquired data useful. Such a system facilitates encoding, storage, and computer processing of data. We present a practical classification of congenital cerebral , cerebellar, and spinal malformations. Our classification is based on the morphologic abnormalities shown by MR and on the time at which the derangement of neural development occurred. A classification based on etiology is not as valuable because the various presumed causes rarely lead to a specific pattern of malformations. The abnor malities reflect the time the noxious agent interfered with neural development, rather than the nature of the noxious agent. The vulnerability of the various structures to adverse agents is greatest during the period of most active growth and development. -

2472 - in Our Physical Examination, 6 Cm Cervical After an Hour, Patient Had Normal Delivery
ACRANIA IN TERM FETUSA Termde fetusta akrani İLKER KAHRAMANOĞLU, MERVE BAKTIROĞLU, FATMA FERDA VERİT Süleymaniye Kadın Hastalıkları Ve Doğum,kadın Hastalıkları Ve Doğum,istanbul, Türkiye Suleymaniye Birth And Women Health Education And Research Hospital, Department Of Obstetrics And Gynecology,istanbul,turkey ÖZET Fetal akrani, kraniyal çatı kemiklerinin kısmi veya tam yokluğu ile karakterize, nadir görülen,yaşam şansı çok düşük olan konjenital bir patolojidir. Takipsiz gebelerde ve tanısı konup terminasyon istemeyen hastalarda maternal invazif girişimin ve aileye psikolojik zararın arttığı, maliyetin yükseldiği göz önünde tutulmalıdır. 2. trimesterde fetal akrani tanısı konulup terminasyon önerilen, aile olarak terminasyonu kabul etmeyen, miadında normal doğum yapan gebe sunulmuştur. Anahtar Kelimeler: Akrani, nöral tüp defekti, term fetus ABSTRACT Acrania is a rare fetal malformation, characterized by abnormal development of the calvarium with a large mass of disorganized brain tissue. Early diagnosis of acrania is important for determining the necessity to terminate the pregnancy and also to decrease the negative psychological and financial effects on patients and doctors. In this report, we present a case of term acrania, diagnosed in second trimester, refused termination because of religious beliefs. Key words: Acrania, neural tube defect, term fetus INTRODUCTİON Neural tube defects cover a broad spectrum early diagnosis of fetal acrania (3, 4). In of morphologic anomalies of the central this report, we present a case of term nervous -

Surveillance for Anencephaly and Spina Bifida and the Impact of Prenatal Diagnosis —United States, 1985–1994
August 25, 1995 / Vol. 44 / No. SS-4 CDC Surveillance Summaries Surveillance for Anencephaly and Spina Bifida and the Impact of Prenatal Diagnosis —United States, 1985–1994 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Centers for Disease Control and Prevention (CDC) Atlanta, Georgia 30333 The MMWR series of publications is published by the Epidemiology Program Office, Centers for Disease Control and Prevention (CDC), Public Health Service, U.S. Depart- ment of Health and Human Services, Atlanta, GA 30333. SUGGESTED CITATION General: Centers for Disease Control and Prevention. CDC Surveillance Sum- maries, August 25, 1995. MMWR 1995;44(No. SS-4). Specific: [Author(s)]. [Title of particular article]. In: CDC Surveillance Sum- maries, August 25, 1995. MMWR 1995;44(No. SS-4):[inclusive page numbers]. Centers for Disease Control and Prevention .......................... David Satcher, M.D., Ph.D. Director The production of this report as an MMWR serial publication was coordinated in: Epidemiology Program Office.................................... Stephen B. Thacker, M.D., M.Sc. Director Richard A. Goodman, M.D., M.P.H. Editor, MMWR Series Scott F. Wetterhall, M.D., M.P.H. Associate Editor, CDC Surveillance Summaries Scientific Information and Communications Program CDC Surveillance Summaries ...................................... Suzanne M. Hewitt, M.P.A. Managing Editor Rachel J. Wilson Ava W. Navin, M.A. Project Editors Peter M. Jenkins Visual Information Specialist Use of trade names and commercial sources is for identification only and does not imply endorsement by the Public Health Service or the U.S. Department of Health and Human Services. Copies can be purchased from Superintendent of Documents, U.S. -

Massachusetts Birth Defects 2011-2012
Massachusetts Birth Defects 2011-2012 Massachusetts Birth Defects Monitoring Program Bureau of Family Health and Nutrition Massachusetts Department of Public Health May 2016 Charles D. Baker, Governor Karyn E. Polito, Lieutenant Governor Marylou Sudders, Secretary, Executive Office of Health and Human Services Monica Bharel, MD, MPH, Commissioner, Massachusetts Department of Public Health Ron Benham, Director, Bureau of Family Health and Nutrition, Acting Director, Massachusetts Center for Birth Defects Research and Prevention Acknowledgements This report was prepared by Rebecca Liberman and Cathleen Higgins, Massachusetts Center for Birth Defects Research and Prevention. We would like to thank the Massachusetts Center for Birth Defects Research and Prevention staff who contributed to this report, including: Marlene Anderka, Xiaoli Chen, Dominique Heinke, Angela Lin, and Gerlinde Munshi. Data in this report have been collected through the efforts of Center field staff, including: Roberta Aucoin, Mitcheka Jalali, Washa Liu, Daniel Sexton, Lori Tetrault and Ashley Tracey. We would like to acknowledge the following individuals and organizations for their time and commitment in supporting the Center: Lewis Holmes, MD, MassGeneral Hospital for Children Carol Louik, ScD, Slone Epidemiology Center, Boston University Allen Mitchell, MD, Slone Epidemiology Center, Boston University Martha Werler, ScD, Department of Epidemiology, Boston University School of Public Health Ed Doherty, March of Dimes Massachusetts Chapter Massachusetts Registry of -

MR Imaging of Fetal Head and Neck Anomalies
Neuroimag Clin N Am 14 (2004) 273–291 MR imaging of fetal head and neck anomalies Caroline D. Robson, MB, ChBa,b,*, Carol E. Barnewolt, MDa,c aDepartment of Radiology, Children’s Hospital Boston, 300 Longwood Avenue, Harvard Medical School, Boston, MA 02115, USA bMagnetic Resonance Imaging, Advanced Fetal Care Center, Children’s Hospital Boston, Harvard Medical School, 300 Longwood Avenue, Boston, MA 02115, USA cFetal Imaging, Advanced Fetal Care Center, Children’s Hospital Boston, Harvard Medical School, 300 Longwood Avenue, Boston, MA 02115, USA Fetal dysmorphism can occur as a result of var- primarily used for fetal MR imaging. When the fetal ious processes that include malformation (anoma- face is imaged, the sagittal view permits assessment lous formation of tissue), deformation (unusual of the frontal and nasal bones, hard palate, tongue, forces on normal tissue), disruption (breakdown of and mandible. Abnormalities include abnormal promi- normal tissue), and dysplasia (abnormal organiza- nence of the frontal bone (frontal bossing) and lack of tion of tissue). the usual frontal prominence. Abnormal nasal mor- An approach to fetal diagnosis and counseling of phology includes variations in the size and shape of the parents incorporates a detailed assessment of fam- the nose. Macroglossia and micrognathia are also best ily history, maternal health, and serum screening, re- diagnosed on sagittal images. sults of amniotic fluid analysis for karyotype and Coronal images are useful for evaluating the in- other parameters, and thorough imaging of the fetus tegrity of the fetal lips and palate and provide as- with sonography and sometimes fetal MR imaging. sessment of the eyes, nose, and ears. -
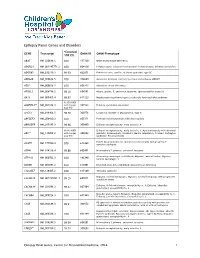
Epilepsy Panel Genes and Disorders
Epilepsy Panel Genes and Disorders *Covered GENE Transcript OMIM ID OMIM Phenotype 10X (%) ABAT NM_020686.5 100 137150 GABA-transaminase deficiency ADGRG1 NM_001145771.2 100 604110 Polymicrogyria, bilateral frontoparietal; Polymicrogyria, bilateral perisylvian ADGRV1 NM_032119.3 99.93 602851 Febrile seizures, familial, 4; Usher syndrome, type 2C ADRA2B NM_000682.5 100 104260 Autosomal dominant cortical myoclonus and epilepsy (ADCME) ADSL NM_000026.2 100 608222 Adenylosuccinase deficiency AFG3L2 NM_006796.2 98.16 604581 Ataxia, spastic, 5, autosomal recessive; Spinocerebellar ataxia 28 AKT3 NM_005465.4 99.97 611223 Megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndrome 93.05 (100% ALDH7A1** NM_001182.3 with Sanger 107323 Epilepsy, pyridoxine-dependent Gap fill) ALG13 NM_018466.5 98.76 300776 Congenital disorder of glycosylation, type Is ARFGEF2 NM_006420.2 100 605371 Periventricular heterotopia with microcephaly ARHGEF9 NM_015185.3 99.96 300430 Epileptic encephalopathy, early infantile, 8 98.96 (100% Epileptic encephalopathy, early infantile, 1; Hydranencephaly with abnormal ARX** NM_139058.2 with Sanger 300382 genitalia; Lissencephaly, X-linked 2; Mental retardation, X-linked; Partington Gap fill) syndrome; Proud syndrome ASAH1 NM_177924.3 613468 Farber lipogranulomatosis; Spinal muscular atrophy with progressive 100 myoclonic epilepsy ASPM NM_018136.4 99.88 605481 Microcephaly 5, primary, autosomal recessive ATP1A2 NM_000702.3 182340 Alternating hemiplegia of childhood; Migraine, familial basilar; Migraine, 100 familial hemiplegic, -

Chapter III: Case Definition
NBDPN Guidelines for Conducting Birth Defects Surveillance rev. 06/04 Appendix 3.5 Case Inclusion Guidance for Potentially Zika-related Birth Defects Appendix 3.5 A3.5-1 Case Definition NBDPN Guidelines for Conducting Birth Defects Surveillance rev. 06/04 Appendix 3.5 Case Inclusion Guidance for Potentially Zika-related Birth Defects Contents Background ................................................................................................................................................. 1 Brain Abnormalities with and without Microcephaly ............................................................................. 2 Microcephaly ............................................................................................................................................................ 2 Intracranial Calcifications ......................................................................................................................................... 5 Cerebral / Cortical Atrophy ....................................................................................................................................... 7 Abnormal Cortical Gyral Patterns ............................................................................................................................. 9 Corpus Callosum Abnormalities ............................................................................................................................. 11 Cerebellar abnormalities ........................................................................................................................................ -

Neuropathology Review.Pdf
Neuropathology Review Richard A. Prayson, MD Humana Press Contents NEUROPATHOLOGY REVIEW 2 Contents Contents NEUROPATHOLOGY REVIEW By RICHARD A. PRAYSON, MD Department of Anatomic Pathology Cleveland Clinic Foundation, Cleveland, OH HUMANA PRESS TOTOWA, NEW JERSEY 4 Contents © 2001 Humana Press Inc. 999 Riverview Drive, Suite 208 Totowa, New Jersey 07512 For additional copies, pricing for bulk purchases, and/or information about other Humana titles, contact Humana at the above address or at any of the following numbers: Tel.: 973-256-1699; Fax: 973-256-8341; E-mail:[email protected]; Website: http://humanapress.com All rights reserved. No part of this book may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, microfilming, recording, or otherwise without written permission from the Publisher. Due diligence has been taken by the publishers, editors, and authors of this book to assure the accuracy of the information published and to describe generally accepted practices. The contributors herein have carefully checked to ensure that the drug selections and dosages set forth in this text are accurate and in accord with the standards accepted at the time of publication. Notwithstanding, as new research, changes in government regulations, and knowledge from clinical experience relating to drug therapy and drug reactions constantly occurs, the reader is advised to check the product information provided by the manufacturer of each drug for any change in dosages or for additional warnings and contraindications. This is of utmost importance when the recommended drug herein is a new or infrequently used drug. It is the responsibility of the treating physician to determine dosages and treatment strategies for individual patients.