Psoriasis, Pityriasis Alba, and Vitiligo
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Melanocytes and Their Diseases
Downloaded from http://perspectivesinmedicine.cshlp.org/ on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press Melanocytes and Their Diseases Yuji Yamaguchi1 and Vincent J. Hearing2 1Medical, AbbVie GK, Mita, Tokyo 108-6302, Japan 2Laboratory of Cell Biology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892 Correspondence: [email protected] Human melanocytes are distributed not only in the epidermis and in hair follicles but also in mucosa, cochlea (ear), iris (eye), and mesencephalon (brain) among other tissues. Melano- cytes, which are derived from the neural crest, are unique in that they produce eu-/pheo- melanin pigments in unique membrane-bound organelles termed melanosomes, which can be divided into four stages depending on their degree of maturation. Pigmentation production is determined by three distinct elements: enzymes involved in melanin synthesis, proteins required for melanosome structure, and proteins required for their trafficking and distribution. Many genes are involved in regulating pigmentation at various levels, and mutations in many of them cause pigmentary disorders, which can be classified into three types: hyperpigmen- tation (including melasma), hypopigmentation (including oculocutaneous albinism [OCA]), and mixed hyper-/hypopigmentation (including dyschromatosis symmetrica hereditaria). We briefly review vitiligo as a representative of an acquired hypopigmentation disorder. igments that determine human skin colors somes can be divided into four stages depend- Pinclude melanin, hemoglobin (red), hemo- ing on their degree of maturation. Early mela- siderin (brown), carotene (yellow), and bilin nosomes, especially stage I melanosomes, are (yellow). Among those, melanins play key roles similar to lysosomes whereas late melanosomes in determining human skin (and hair) pigmen- contain a structured matrix and highly dense tation. -

Update on Challenging Disorders of Pigmentation in Skin of Color Heather Woolery-Lloyd, M.D
Update on Challenging Disorders of Pigmentation in Skin of Color Heather Woolery-Lloyd, M.D. Director of Ethnic Skin Care Voluntary Assistant Professor Miller/University of Miami School of Medicine Department of Dermatology and Cutaneous Surgery What Determines Skin Color? What Determines Skin Color? No significant difference in the number of melanocytes between the races 2000 epidermal melanocytes/mm2 on head and forearm 1000 epidermal melanocytes/mm2 on the rest of the body differences present at birth Jimbow K, Quevedo WC, Prota G, Fitzpatrick TB (1999) Biology of melanocytes. In I. M. Freedberg, A.Z. Eisen, K. Wolff,K.F. Austen, L.A. Goldsmith, S. I. Katz, T. B. Fitzpatrick (Eds.), Dermatology in General Medicine 5th ed., pp192-220, New York, NY: McGraw Hill Melanosomes in Black and White Skin Black White Szabo G, Gerald AB, Pathak MA, Fitzpatrick TB. Nature1969;222:1081-1082 Jimbow K, Quevedo WC, Prota G, Fitzpatrick TB (1999) Biology of melanocytes. In I. M. Freedberg, A.Z. Eisen, K. Wolff, K.F. Austen, L.A. Goldsmith, S. I. Katz, T. B. Fitzpatrick (Eds.), Dermatology in General Medicine 5th ed., pp192- 220, New York, NY: McGraw Hill Role of Melanin-Advantages Melanin absorbs and scatters energy from UV and visible light to protect epidermal cells from UV damage Disadvantages Inflammation or injury to the skin is almost immediately accompanied by alteration in pigmentation Hyperpigmentation Hypopigmentation Dyschromias Post-Inflammatory hyperpigmentation Acne Melasma Lichen Planus Pigmentosus Progressive Macular Hypomelanosis -

Frequency of Different Types of Facial Melanoses Referring to the Department of Dermatology and Venereology, Nepal Medical Colle
Amatya et al. BMC Dermatology (2020) 20:4 https://doi.org/10.1186/s12895-020-00100-3 RESEARCH ARTICLE Open Access Frequency of different types of facial melanoses referring to the Department of Dermatology and Venereology, Nepal Medical College and Teaching Hospital in 2019, and assessment of their effect on health-related quality of life Bibush Amatya* , Anil Kumar Jha and Shristi Shrestha Abstract Background: Abnormalities of facial pigmentation, or facial melanoses, are a common presenting complaint in Nepal and are the result of a diverse range of conditions. Objectives: The objective of this study was to determine the frequency, underlying cause and impact on quality of life of facial pigmentary disorders among patients visiting the Department of Dermatology and Venereology, Nepal Medical College and Teaching Hospital (NMCTH) over the course of one year. Methods: This was a cross-sectional study conducted at the Department of Dermatology and Venereology, NMCT H. We recruited patients with facial melanoses above 16 years of age who presented to the outpatient department. Clinical and demographic data were collected and all the enrolled participants completed the validated Nepali version of the Dermatology Life Quality Index (DLQI). Results: Between January 5, 2019 to January 4, 2020, a total of 485 patients were recruited in the study. The most common diagnoses were melasma (166 patients) and post acne hyperpigmentation (71 patients). Quality of life impairment was highest in patients having melasma with steroid induced rosacea-like dermatitis (DLQI = 13.54 ± 1.30), while it was lowest in participants with ephelides (2.45 ± 1.23). Conclusion: Facial melanoses are a common presenting complaint and lead to substantial impacts on quality of life. -

In Dermatology Visit with Me to Discuss
From time to time new treatments surface for any medical field, and the last couple of years have seen new treatments emerge, or new applications for familiar treatments. I wanted to summarize some of these New Therapies widely available remedies and encourage you to schedule a in Dermatology visit with me to discuss. Written by Board Certified Dermatologist James W. Young, DO, FAOCD Nicotinamide a significant reduction in melanoma in Antioxidants Nicotinamide (niacinamide) is a form high risk skin cancer patients at doses Green tea, pomegranate, delphinidin of vitamin B3. The deficiency of vitamin more than 600 and less than 4,000 IU and fisetin are all under current study for daily. B3 causes pellagra, a condition marked either oral or topical use in the reduction by 4D’s – (photo) Dermatitis, Dementia, Polypodium Leucotomos of the incidence of skin cancer, psoriasis Diarrhea and (if left untreated) Death. and other inflammatory disorders. I’ll be Polypodium leucotomos is a Central This deficiency is rare in developed sure to keep patients updated. countries, but is occasionally seen America fern that is available in several in alcoholism, dieting restrictions, or forms, most widely as Fernblock What Are My Own Thoughts? malabsorption syndromes. Nicotinamide (Amazon) or Heliocare (Walgreen’s and I take Vitamin D 1,000 IU and Heliocare does not cause the adverse effects of Amazon) and others. It is an antioxidant personally. Based on new research, I Nicotinic acid and is safe at doses up to that reduces free oxygen radicals and have also added Nicotinamide which 3,000mg daily. may reduce inflammation in eczema, dementia, sunburn, psoriasis, and vitiligo. -

Psoriasis and Vitiligo: an Association Or Coincidence?
igmentar f P y D l o is a o n r r d e u r o s J Solovan C, et al., Pigmentary Disorders 2014, 1:1 Journal of Pigmentary Disorders DOI: 10.4172/jpd.1000106 World Health Academy ISSN: 2376-0427 Letter To Editor Open Access Psoriasis and Vitiligo: An Association or Coincidence? Caius Solovan1, Anca E Chiriac2, Tudor Pinteala2, Liliana Foia2 and Anca Chiriac3* 1University of Medicine and Pharmacy “V Babes” Timisoara, Romania 2University of Medicine and Pharmacy “Gr T Popa” Iasi, Romania 3Apollonia University, Nicolina Medical Center, Iasi, Romania *Corresponding author: Anca Chiriac, Apollonia University, Nicolina Medical Center, Iasi, Romania, Tel: 00-40-721-234-999; E-mail: [email protected] Rec date: April 21, 2014; Acc date: May 23, 2014; Pub date: May 25, 2014 Citation: Solovan C, Chiriac AE, Pinteala T, Foia L, Chiriac A (2014) Psoriasis and Vitiligo: An Association or Coincidence? Pigmentary Disorders 1: 106. doi: 10.4172/ jpd.1000106 Copyright: © 2014 Solovan C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Letter to Editor Dermatitis herpetiformis 1 0.08% Sir, Chronic urticaria 2 0.16% The worldwide occurrence of psoriasis in the general population is Lyell syndrome 1 0.08% about 2–3% and of vitiligo is 0.5-1%. Coexistence of these diseases in the same patient is rarely reported and based on a pathogenesis not Quincke edema 1 0.08% completely understood [1]. -

Pityriasis Alba Revisited: Perspectives on an Enigmatic Disorder of Childhood
Pediatric ddermatologyermatology Series Editor: Camila K. Janniger, MD Pityriasis Alba Revisited: Perspectives on an Enigmatic Disorder of Childhood Yuri T. Jadotte, MD; Camila K. Janniger, MD Pityriasis alba (PA) is a localized hypopigmented 80 years ago.2 Mainly seen in the pediatric popula- disorder of childhood with many existing clinical tion, it primarily affects the head and neck region, variants. It is more often detected in individuals with the face being the most commonly involved with a darker complexion but may occur in indi- site.1-3 Pityriasis alba is present in individuals with viduals of all skin types. Atopy, xerosis, and min- all skin types, though it is more noticeable in those with eral deficiencies are potential risk factors. Sun a darker complexion.1,3 This condition also is known exposure exacerbates the contrast between nor- as furfuraceous impetigo, erythema streptogenes, mal and lesional skin, making lesions more visible and pityriasis streptogenes.1 The term pityriasis alba and patients more likely to seek medical atten- remains accurate and appropriate given the etiologic tion. Poor cutaneous hydration appears to be a elusiveness of the disorder. common theme for most riskCUTIS factors and may help elucidate the pathogenesis of this disorder. The Epidemiology end result of this mechanism is inappropriate mel- Pityriasis alba primarily affects preadolescent children anosis manifesting as hypopigmentation. It must aged 3 to 16 years,4 with onset typically occurring be differentiated from other disorders of hypopig- between 6 and 12 years of age.5 Most patients are mentation, such as pityriasis versicolor alba, vitiligo, younger than 15 years,3 with up to 90% aged 6 to nevus depigmentosus, and nevus anemicus. -

A New Insight on Atopic Skin Diathesis: Is It Correlated with the Severity of Melasma
A New Insight on Atopic Skin Diathesis: Is It Correlated with the Severity of Melasma Danar Wicaksono1*, Rima Mustafa2, Sri Awalia Febriana1, Kristiana Etnawati1 1 Dermatovenereology Department, Faculty of Medicine Universitas Gadjah Mada – Dr. Sardjito General Hospital, Yogyakarta-Indonesia 2 Clinical Epidemiology and Biostatistics Unit, Faculty of Medicine Universitas Gadjah Mada –Dr. Sardjito General Hospital, Yogyakarta-Indonesia Keywords: Melasma, atopic skin diathesis (ASD), MASI score, atopic dermatitis (AD) Abstract: Melasma is a macular lesion of light brown to dark on the sun-exposed area, especially on the face. Atopic Skin Diathesis (ASD) is a clinical term to describe skin atopics with previous, present or future atopic dermatitis (AD). Dennie-Morgan infraorbital folds are secondary creases in the skin below the lower eyelids with a sensitivity of 78% and a specificity of 76% to diagnose AD. Melasma skin is characterized by impaired stratum corneum integrity and a delayed barrier recovery rate. Barrier dysfunction will stimulate keratinocyte to secrete keratinocyte-derived factor, which plays role in skin pigmentation process in melasma. To analyze correlation between ASD and Melasma Area Severity Index (MASI) score in melasma patient. This study is an observational analytic study with cross sectional design. Measurement of ASD and MASI score were done in 60 subjects with melasma who went to dermatology outpatient clinic Dr. Sardjito General Hospital from July 2017 to Januari 2018. The correlation between ASD and MASI score was analyzed using Pearson correlation. The result of this study showed no significant correlation between ASD and MASI scores (r: 0.02, p: 0,85). Crude Relative Risk (RR) for Dennie-Morgan infraorbital folds and MASI score was 4 (1.01-15.87). -

Pigmented Contact Dermatitis and Chemical Depigmentation
18_319_334* 05.11.2005 10:30 Uhr Seite 319 Chapter 18 Pigmented Contact Dermatitis 18 and Chemical Depigmentation Hideo Nakayama Contents ca, often occurs without showing any positive mani- 18.1 Hyperpigmentation Associated festations of dermatitis such as marked erythema, with Contact Dermatitis . 319 vesiculation, swelling, papules, rough skin or scaling. 18.1.1 Classification . 319 Therefore, patients may complain only of a pigmen- 18.1.2 Pigmented Contact Dermatitis . 320 tary disorder, even though the disease is entirely the 18.1.2.1 History and Causative Agents . 320 result of allergic contact dermatitis. Hyperpigmenta- 18.1.2.2 Differential Diagnosis . 323 tion caused by incontinentia pigmenti histologica 18.1.2.3 Prevention and Treatment . 323 has often been called a lichenoid reaction, since the 18.1.3 Pigmented Cosmetic Dermatitis . 324 presence of basal liquefaction degeneration, the ac- 18.1.3.1 Signs . 324 cumulation of melanin pigment, and the mononucle- 18.1.3.2 Causative Allergens . 325 ar cell infiltrate in the upper dermis are very similar 18.1.3.3 Treatment . 326 to the histopathological manifestations of lichen pla- 18.1.4 Purpuric Dermatitis . 328 nus. However, compared with typical lichen planus, 18.1.5 “Dirty Neck” of Atopic Eczema . 329 hyperkeratosis is usually milder, hypergranulosis 18.2 Depigmentation from Contact and saw-tooth-shape acanthosis are lacking, hyaline with Chemicals . 330 bodies are hardly seen, and the band-like massive in- 18.2.1 Mechanism of Leukoderma filtration with lymphocytes and histiocytes is lack- due to Chemicals . 330 ing. 18.2.2 Contact Leukoderma Caused Mainly by Contact Sensitization . -

Alopecia Areata, Atopic Dermatitis and Vitiligo (Global) Competitive Grant Program - Internal Pfizer Review Process
Pfizer Announces a Research Grant RFP Alopecia Areata, Atopic Dermatitis and Vitiligo (Global) Competitive Grant Program - internal Pfizer review process I. Background Pfizer Global Medical Grants (GMG) supports the global healthcare community’s independent initiatives (e.g., research, quality improvement, or education) to improve patient outcomes in areas of unmet medical need that are aligned with Pfizer’s medical and/or scientific strategies. Pfizer’s GMG competitive grant program involves a publicly posted general Request for Proposal (RFP) that provides detail regarding a general area of interest, sets timelines for review and approval, and uses an internal Pfizer review process to make final grant decisions. Organizations are invited to submit an application addressing the research gaps as outlined in the specific RFP. For all Investigator Sponsored Research (ISRs) and general research grants, the grant requester (and ultimately the grantee) is responsible for the design, implementation, sponsorship, and conduct of the independent initiative supported by the grant, including compliance with any regulatory requirements. Pfizer must not be involved in any aspect of study protocol or project development, nor the conduct or monitoring of the research program. Alopecia3 Areata, Atopic Dermatitis and Vitiligo (Global) II. Eligibility Geographic Scope: Global (including U.S.A.) Applicant Eligibility • Only organizations are eligible to receive grants, not individuals or Criteria medical practice groups. • The applicant (PI) must have a -

SKIN HYPERPIGMENTATION DISORDERS and USE of HERBAL EXTRACTS: a REVIEW Priyam Goswami* and H
Current Trends in Pharmaceutical Research 2020 Vol 7 Issue 2 © Dibrugarh University www.dibru.ac.in/ctpr ISSN: 2319-4820 (Print) 2582-4783 (Online) Mini Review SKIN HYPERPIGMENTATION DISORDERS AND USE OF HERBAL EXTRACTS: A REVIEW Priyam Goswami* and H. K. Sharma Department of Pharmaceutical Sciences, Faculty of Science and Engineering, Dibrugarh University Abstract Background: Problems pertaining to the skin pigmentation is one of the major concerns that affect the quality of life of human beings especially women. The disturbances in the melanin production mainly results in skin pigmentation disorders. For centuries natural ingredients have been used in the treatment of skin hyperpigmentation disorders. Since synthetic cosmetic ingredients can have potential side effects, emphasis has been given on the herbal products, which are considered to be mild and biodegradable, exhibiting low toxicity. Objective: The objective of this review is to provide a brief understanding about the skin hyperpigmentation disorders and the use of various herbal extracts as a notable approach for its treatment. Methods: A narrative literature review was conducted with the extraction of information, which was analyzed from various databases viz. Google scholar, Science Direct, PubMed, Wiley Online Library etc., Results and Discussions: The preferences of the people for the use of herbal skin- lightening agents over the synthetic ones have gained wide spread popularity. Potentially active compounds that have been extracted from the plants have been identified which provide scope for its use a novel depigmenting agent. The improvement in the efficacy of the herbal extracts is mainly due to synergism, and hence this property yields great results when used in cosmetic formulations. -
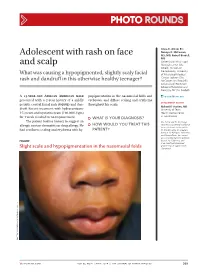
Adolescent with Rash on Face and Scalp
Photo RoUNDS Anna K. Allred, BS; Nancye K. McCowan, Adolescent with rash on face MS, MD; Robert Brodell, MD University of Mississippi and scalp Medical Center (Ms. Allred); Division of Dermatology, University What was causing a hypopigmented, slightly scaly facial of Mississippi Medical Center, Jackson (Drs. rash and dandruff in this otherwise healthy teenager? McCowan and Brodell); University of Rochester School of Medicine and Dentistry, NY (Dr. Brodell) A 13-year-old African American male popigmentation in the nasomesial folds and [email protected] presented with a 2-year history of a mildly eyebrows, and diffuse scaling and erythema DEpartment EDItOR pruritic central facial rash (FIGURE) and dan- throughout his scalp. Richard p. Usatine, MD druff. Recent treatment with hydrocortisone University of Texas 1% cream and nystatin cream (100,000 U/gm) Health Science Center at San Antonio for 1 week resulted in no improvement. ● What is youR diAgnosis? The patient had no history to suggest an Ms. Allred and Dr. McCowan allergic contact dermatitis or drug allergy. He ● HoW Would you TReAT THIS reported no potential conflict of interest relevant to this article. had confluent scaling and erythema with hy- pATIENT? Dr. Brodell serves on speaker’s bureaus for Allergan, Galderma, and PharmaDerm, has served as a consultant and on advisory Figure boards for Galderma, and is an investigator/received grant/research support from Slight scale and hypopigmentation in the nasomesial folds Genentech. PHO T o COU RT ESY OF : R o B e RT BR ODELL , MD jfponline.com Vol 63, no 4 | ApRIL 2014 | The jouRnAl of Family PracTice 209 PHOTO RoUNDS Diagnosis: the Malassezia yeast and topical steroids are Seborrheic dermatitis used to suppress inflammation. -

Pattern of Skin Diseases in a Rural Village Development Community of Nepal Shrestha R1, Lama L2, Gurung D3, Shrestha DP4, Rosdahl I5
Vol. 12, No. 1, 2014 Original Article Pattern Of Skin Diseases In A Rural Village Development Community Of Nepal Shrestha R1, Lama L2, Gurung D3, Shrestha DP4, Rosdahl I5 1,2Clinical Tutor, Department of Dermatology, Abstract National Academy of Medical Sciences, Bir Introduction: Skin diseases are a common cause of morbidity in Hospital, Kathmandu, Nepal; 3Consultant Nepal as per the health services report. There is limited information Dermatologist, DI Skin Hospital and Research on the prevalence and pattern of skin diseases in the community. Center, Kathmandu, Nepal; 4Professor, Department of Dermatology & Venereology, Institute of Objectives: This study was conducted to determine the pattern of Medicine, Maharajgunj Medical Campus, skin diseases in a rural village development community of Nepal. Kathmandu, Nepal; 5Visiting Professor, Department of Dermatology, Kathmandu Medical College, Material and methods: Two dermatologic health camps were Kathmandu, Nepal. conducted, during which, the villagers were examined by dermatologists. The skin diseases diagnosed were recorded in a Address for correspondence proforma. Dr. Dwarika P Shrestha Results: There were 433 individuals examined and 359 (male-47.9%; female-52.1%) had skin disease identified clinically (camp prevalence- Department of Dermatology, IOM, 83%). The age of patients ranged from 1 to 80 years (mean-24.5; Kathmandu, Nepal SD±15.9), with majority in the age group of 10-19 years. The most Telephone: 5592217; common skin disease category was eczemas (36.4%), followed by E-mail: [email protected] infections (28.4%), acne (22%), pigment disorders (34%) and urticaria (12.3%). Citation Conclusion: Skin diseases were common in the community. The Shrestha R, Lama L, Gurung D, Shrestha DP, five most common Skin disease categories were eczemas, infections, Rosdahl I.