Davy and Alkalies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

History of the Chlor-Alkali Industry
2 History of the Chlor-Alkali Industry During the last half of the 19th century, chlorine, used almost exclusively in the textile and paper industry, was made [1] by reacting manganese dioxide with hydrochloric acid 100–110◦C MnO2 + 4HCl −−−−−−→ MnCl2 + Cl2 + 2H2O (1) Recycling of manganese improved the overall process economics, and the process became known as the Weldon process [2]. In the 1860s, the Deacon process, which generated chlorine by direct catalytic oxidation of hydrochloric acid with air according to Eq. (2) was developed [3]. ◦ 450–460 C;CuCl2 cat. 4HCl + O2(air) −−−−−−−−−−−−−−→ 2Cl2 + 2H2O(2) The HCl required for reactions (1) and (2) was available from the manufacture of soda ash by the LeBlanc process [4,5]. H2SO4 + 2NaCl → Na2SO4 + 2HCl (3) Na2SO4 + CaCO3 + 2C → Na2CO3 + CaS + 2CO2 (4) Utilization of HCl from reaction (3) eliminated the major water and air pollution problems of the LeBlanc process and allowed the generation of chlorine. By 1900, the Weldon and Deacon processes generated enough chlorine for the production of about 150,000 tons per year of bleaching powder in England alone [6]. An important discovery during this period was the fact that steel is immune to attack by dry chlorine [7]. This permitted the first commercial production and distribu- tion of dry liquid chlorine by Badische Anilin-und-Soda Fabrik (BASF) of Germany in 1888 [8,9]. This technology, using H2SO4 for drying followed by compression of the gas and condensation by cooling, is much the same as is currently practiced. 17 “chap02” — 2005/5/2 — 09Brie:49 — page 17 — #1 18 CHAPTER 2 In the latter part of the 19th century, the Solvay process for caustic soda began to replace the LeBlanc process. -

A Short History of the Royal Aeronautical Society
A SHORT HISTORY OF THE ROYAL AERONAUTICAL SOCIETY Royal Aeronautical Society Council Dinner at the Science Museum on 26 May 1932 with Guest of Honour Miss Amelia Earhart. Edited by Chris Male MRAeS Royal Aeronautical Society www.aerosociety.com Afterburner Society News RAeS 150th ANNIVERSARY www.aerosociety.com/150 The Royal Aeronautical Society: Part 1 – The early years The Beginning “At a meeting held at Argyll Lodge, Campden Hill, Right: The first Aeronautical on 12 January 1866, His Grace The Duke of Argyll Exhibition, Crystal Palace, 1868, showing the presiding; also present Mr James Glaisher, Dr Hugh Stringfellow Triplane model W. Diamond, Mr F.H. Wenham, Mr James Wm. Butler and other exhibits. No fewer and Mr F.W. Brearey. Mr Glaisher read the following than 77 exhibits were address: collected together, including ‘The first application of the Balloon as a means of engines, lighter- and heavier- than-air models, kites and ascending into the upper regions of the plans of projected machines. atmosphere has been almost within the recollection A special Juror’s Report on on ‘Aerial locomotion and the laws by which heavy of men now living but with the exception of some the exhibits was issued. bodies impelled through air are sustained’. of the early experimenters it has scarcely occupied Below: Frederick W Brearey, Wenham’s lecture is now one of the aeronautical Secretary of the the attention of scientific men, nor has the subject of Aeronautical Society of Great classics and was the beginning of the pattern of aeronautics been properly recognised as a distinct Britain, 1866-1896. -

Suppression Mechanisms of Alkali Metal Compounds
SUPPRESSION MECHANISMS OF ALKALI METAL COMPOUNDS Bradley A. Williams and James W. Fleming Chemistry Division, Code 61x5 US Naval Research Lnhoratory Washington, DC 20375-5342, USA INTRODUCTION Alkali metal compounds, particularly those of sodium and potassium, are widely used as fire suppressants. Of particular note is that small NuHCOi particles have been found to be 2-4 times more effective by mass than Halon 1301 in extinguishing both eountertlow flames [ I] and cup- burner flames [?]. Furthermore, studies in our laboratory have found that potassium bicarbonate is some 2.5 times more efficient by weight at suppression than sodium bicarhonatc. The primary limitation associated with the use of alkali metal compounds is dispersal. since all known compounds have very low volatility and must he delivered to the fire either as powders or in (usually aqueous) solution. Although powders based on alkali metals have been used for many years, their mode of effective- ness has not generally been agreed upon. Thermal effects [3],namely, the vaporization of the particles as well as radiative energy transfer out of the flame. and both homogeneous (gas phase) and heterogeneous (surface) chemistry have been postulated as mechanisms by which alkali metals suppress fires [4]. Complicating these issues is the fact that for powders, particle size and morphology have been found to affect the suppression properties significantly [I]. In addition to sodium and potassium, other alkali metals have been studied, albeit to a consider- ably lesser extent. The general finding is that the suppression effectiveness increases with atomic weight: potassium is more effective than sodium, which is in turn more effective than lithium [4]. -

Alkali-Silica Reactivity: an Overview of Research
SHRP-C-342 Alkali-Silica Reactivity: An Overview of Research Richard Helmuth Construction Technology Laboratories, Inc. With contributions by: David Stark Construction Technology Laboratories, Inc. Sidney Diamond Purdue University Micheline Moranville-Regourd Ecole Normale Superieure de Cachan Strategic Highway Research Program National Research Council Washington, DC 1993 Publication No. SHRP-C-342 ISBN 0-30cL05602-0 Contract C-202 Product No. 2010 Program Manager: Don M. Harriott Project Maxtager: Inam Jawed Program AIea Secretary: Carina Hreib Copyeditor: Katharyn L. Bine Brosseau May 1993 key words: additives aggregate alkali-silica reaction cracking expansion portland cement concrete standards Strategic Highway Research Program 2101 Consti!ution Avenue N.W. Washington, DC 20418 (202) 334-3774 The publicat:Lon of this report does not necessarily indicate approval or endorsement by the National Academy of Sciences, the United States Government, or the American Association of State Highway and Transportation Officials or its member states of the findings, opinions, conclusions, or recommendations either inferred or specifically expressed herein. ©1993 National Academy of Sciences 1.5M/NAP/593 Acknowledgments The research described herein was supported by the Strategic Highway Research Program (SHRP). SHRP is a unit of the National Research Council that was authorized by section 128 of the Surface Transportation and Uniform Relocation Assistance Act of 1987. This document has been written as a product of Strategic Highway Research Program (SHRP) Contract SHRP-87-C-202, "Eliminating or Minimizing Alkali-Silica Reactivity." The prime contractor for this project is Construction Technology Laboratories, with Purdue University, and Ecole Normale Superieure de Cachan, as subcontractors. Fundamental studies were initiated in Task A. -

The Early History of Catalysis
The Early History of Catalysis By Professor A. J. B. Robertson Department of Chemistry, King’s College, London One hundred and forty years ago it was Berzelius proceeded to propose the exist- possible for one man to prepare an annual ence of a new force which he called the report on the progress of the whole of “catalytic force” and he called “catalysis” the chemistry, and for many years this task was decomposition of bodies by this force. This undertaken by the noted Swedish chemist is probably the first recognition of catalysis J. J. Berzelius for the Stockholm Academy of as a wide-ranging natural phenomenon. Sciences. In his report submitted in 1835 and Metallic catalysts had in fact been used in published in 1836 Berzelius reviewed a num- the laboratory before 1800 by Joseph Priestley, ber of earlier findings on chemical change in the discoverer of oxygen, and by the Dutch both homogeneous and heterogeneous sys- chemist Martinus van Marum, both of whom tems, and showed that these findings could be made observations on the dehydrogenation of rationally co-ordinated by the introduction alcohol on metal catalysts. However, it seems of the concept of catalysis. In a short paper likely that these investigators regarded the summarising his ideas on catalysis as a new metal merely as a source of heat. In 1813, force, he wrote (I): Louis Jacques Thenard discovered that ammonia is decomposed into nitrogen and “It is, then, proved that several simple or compound bodies, soluble and insoluble, have hydrogen when passed over various red-hot the property of exercising on other bodies an metals, and ten years later, with Pierre action very different from chemical affinity. -

Evidence Synthesis on the EU-UK Relationship on Research and Innovation January 2018
Evidence synthesis on the EU-UK relationship on research and innovation January 2018 1. Introduction The Royal Society and the Wellcome Trust have undertaken a rapid evidence synthesis on the EU-UK research and innovation relationship as part of their Future Partnership Project. Organisations and individuals were invited to submit evidence and analyses for inclusion. Evidence was also gathered through internet searches to ensure an inclusive approach. The Annex is a summary of the methods. Two questions were used in gathering evidence and in determining the material in scope: 1. What incentives, infrastructure and mechanisms can be accessed by research and innovation organisations, funders and individuals in Member States to support collaborations? 2. How do Member States currently use and benefit from these and how might they be affected by Brexit? This paper is a synthesis of the evidence and covers funding, infrastructures, mobility, collaboration and regulation, with a focus on links between the EU and the UK. 2. Overview of the evidence base A few major reports were of particular relevance; the Royal Society’s three reports on the role of the EU in UK research and innovation and two reports commissioned from Technopolis Group by UK organisations, on the role of EU funding in UK research and innovation and the impact of collaboration: the value of UK medical research to EU science and health1,2. These documents were often referenced in other submissions. A report from the Lords Science and Technology Committee’s inquiry on EU Membership and UK Science also summarises many sources of evidence relevant to this synthesis. -
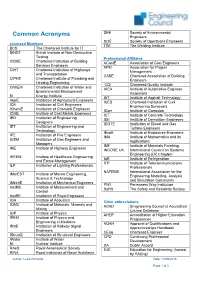
Common Acronyms Engineers SOE Society of Operations Engineers Licensed Members TWI the Welding Institute BCS the Chartered Institute for IT
SEE Society of Environmental Common Acronyms Engineers SOE Society of Operations Engineers Licensed Members TWI The Welding Institute BCS The Chartered Institute for IT BINDT British Institute of Non-Destructive Testing Professional Affiliates CIBSE Chartered Institution of Building ACostE Association of Cost Engineers Services Engineers APM Association for Project CIHT Chartered Institution of Highways Management and Transportation CABE Chartered Association of Building CIPHE Chartered Institute of Plumbing and Engineers Heating Engineering CQI Chartered Quality Institute CIWEM Chartered Institution of Water and IAEA Institute of Automotive Engineer Environmental Management Assessors EI Energy Institute IAT Institute of Asphalt Technology IAgrE Institution of Agricultural Engineers ICES Chartered Institution of Civil ICE Institution of Civil Engineers Engineering Surveyors IChemE Institution of Chemical Engineers ICorr Institute of Corrosion ICME Institute of Cast Metals Engineers ICT Institute of Concrete Technology IED Institution of Engineering IDE Institute of Demolition Engineers Designers IDGTE Institution of Diesel and Gas IET Institution of Engineering and Turbine Engineers Technology IExpE Institute of Explosives Engineers IFE Institution of Fire Engineers IMA Institute of Mathematics and its IGEM Institution of Gas Engineers and Applications Managers IMF Institute of Materials Finishing IHE Institute of Highway Engineers INCOSE UK International Council on Systems Engineering (UK Chapter) IHEEM Institute of Healthcare Engineering -

1 3.1 What Is Heat?
Last revised 30/03/2021 3.1 What is heat? Curriculum links ACSSU155 Energy appears in different forms, including movement (kinetic energy), heat and potential energy, and energy transformations and transfers cause change within systems. KEY IDEAS • Energy isn’t stuff. • Energy looks different in different situations – it can be transferred from one object to another. • The temperature of an object can be raised by doing work on it (e.g. friction). • Temperature is related to the movement (kinetic energy) of the particles in a substance. ACSHE226 Science knowledge can develop through collaboration across the disciplines of science and the contributions of people from a range of cultures. Lesson outcomes At the end of this activity students will be able to: • describe how Count Rumford’s observations provided evidence against the caloric theory of heat. What ideas might your students already have? • Students will have a general understanding that heat flows from hot objects to cold. This will be covered in Activity 3.3. The common use of the word flow is unfortunate, as it suggests a fluid. Key vocabulary Heat Teacher content information It is not the most desirable approach to introduce weapons of war as teaching tools, but this particular experiment was a fundamental and famous one, which transformed our understanding of heat and energy. Cannons originated in ancient China along with the gun powder required to fire them. They were important in warfare for many centuries and modern artillery guns are simply a later modification. Only recently have powered missiles replaced modern artillery. Cannons were traditionally made by casting in solid metal, usually bronze or iron. -

Lectures 7-8 Thurs 23.Iv.09 HAS 222D Introduction to Energy
Lectures 7-8 Thurs 23.iv.09 HAS 222d Introduction to energy & environment Atmosphere-ocean: circulation The atmosphere/ocean system is a ‘heat engine’ largely driven by the sun…that is, a contraption in which heated fluid (air or water) expands and, under gravity, becomes buoyant and rises. The ‘input and output’ temperatures differ by very roughly 300C and so the heat engine cannot convert more than about 10% of the heat flow to the Earth into mechanical energy of the circulation (10% is from the Carnot efficiency, (T -T )/T using 300K and 270K as the absolute temperatures 1 2 1 meet: Benjamin Thompson (Count Rumford) 1753-1814 http://www.rumford.com/Rumford.html determining the conversion of mechanical energy into thermal energy (‘heat’) A cannon barrel is bored from solid iron by a pair of horses connected to an auger...(drill)…Rumford built a box around the barrel, filled it with water and kept track of the rising water temperature. This established he equivalence of thermal energy and a known about of mechanical energy (exerted at a rate of 2 horsepower!) Rumford Company - Baking Powder - 10 oz by Rumford Company Buy new: $2.39 In Stock (3) Grocery: See all 4 items The origin of the Rumford brand name is traced to Count Rumford (Benjamin “James” Thompson of Woburn, Massachusetts), a gifted inventor and scientist. Thompson, who is said to have bootlegged physics courses at Harvard when still a poor boy, became one of the discoverers of the Law of Conservation of Energy, and left the endowment for the Rumford Professorship in 1814. -

Wellcome Trust Annual Report and Financial Statements 2018 Is © the Wellcome Trust and Is Licensed Under Creative Commons Attribution 2.0 UK
Annual Report and Financial Statements 2018 Table of contents Report from Chair 3 Report from Director 4 Trustee’s Report 5 What we do 6 Review of Charitable Activities 7 Review of Investment Activities 18 Financial Review 28 Structure and Governance 33 Social Responsibility 38 Risk Management 40 Remuneration Report 42 Nomination Committee Report 44 Remuneration Committee Report 45 Investment Committee Report 46 Audit and Risk Committee Report 47 Independent Auditor’s Report 49 Financial Statements 59 Consolidated Statement of Financial Activities 60 Consolidated Balance Sheet 61 Statement of Financial Activities of the Trust 62 Balance Sheet of the Trust 63 Consolidated Cash Flow Statement 64 Notes to the Financial Statements 65 Reference and Administrative Details 116 2 Table of Contents Wellcome Trust Annual Report 2018 Report from Chair In March 2018, the Board of Governors and I year, driven by the performance of the investment decided to reappoint Jeremy Farrar as Director portfolio, which returned 13.4%, or 10.7% after of Wellcome for a further five years. Under his inflation. This year has seen a return of market leadership, Wellcome is ambitious, innovative and volatility as the economic cycle has matured. willing to take risks in pursuit of our mission of Central Banks, led by the US Federal Reserve, have improving health by helping great ideas to thrive. begun to raise interest rates and remove the prop to Wellcome’s work is underpinned by the financial asset prices previously provided by quantitative strength we derive from our £25.9 billion investment easing. Uncertainties about Brexit have generated portfolio. -

Royal Society, 1985
The Public Understanding of Science Report of a Royal Society adhoc Group endorsed by the Council of the Royal Society The Royal Society 6 Carlton House Terrace London SWlY 5AG CONTENTS Page Preface 5 Summary 6 1. Introduction 7 2. Why it matters 9 3. The present position 12 4. Formal education 17 5. The mass media 2 1 6. ' The scientific community 24 7. Public lectures, children's activities, museums and libraries 27 8. Industry 29 9. Conclusions and recommendations 31 Annexes A. List of those submitting evidence B. Visits and seminars C. Selected bibliography PREFACE This report was prepared by an ad hoc group under the chairmanship of Dr W.F. Bodmer, F.R.S.; it has been endorsed by the Council of the Royal Society. It deals with an issue that is important not only, or even mainly, for the scientific community but also for the nation as a whole and for each individual within it. More than ever, people need some understanding of science, whether they are involved in decision-making at a national or local level, in managing industrial companies, in skilled or semi-skilled employment, in voting as private citizens or in making a wide range of personal decisions. In publishing this report the Council hopes that it will highlight this need for an overall awareness of the nature of science and, more particularly, of the way that science and technology pervade modern life, and that it will generate both debate and decisions on how best they can be fostered. The report makes a number of recommendations. -

Biography: Sir Benjamin Thompson, Count Rumford
Biography: Sir Benjamin Thompson, Count Rumford Much of what we know today about heat began with the ideas of Count Rumford which he developed in the late eighteenth century in Munich, Germany. That, how- ever, is not all for which Rumford is famous. He originated the study of human nutri- tion and the insulating properties of clothing, created the soup kitchen, and invented thermal underwear, the coffee percolator, the kitchen oven, and central heating, to mention but a few of his many innovations. Rumford was not born with that name and not in Germany. In 1753, in the town of Woburn, Massachusetts, USA, Ruth and Benjamin Thompson became the proud parents of a baby boy, whom they named Benjamin. This biography is about Benjamin who, at the age of 39, became Count Rumford of the Holy Roman Empire. Benjamin’s father died when the child was only two mation to the British. Local activist groups fighting years old. His mother’s hasty remarriage provided Ben- against British rule accused him of being a British spy jamin with a stepfather whom he disliked. Fortunately, and pursued him. When an angry mob came to his unlike most other children his age, Benjamin was sent home, he was already well on his way to Boston. Soon, to grammar school at age eight. By age 13, having ac- it grew too risky for British loyalists to remain in Amer- quired a mastery of advanced algebra, geometry, as- ica, and, in April of 1776, Thomson was evacuated to tronomy, and even higher mathematics, he left school London, England.