Humphry Davy, Nitrous Oxide, the Pneumatic Institution, and the Royal Institution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Short History of the Royal Aeronautical Society
A SHORT HISTORY OF THE ROYAL AERONAUTICAL SOCIETY Royal Aeronautical Society Council Dinner at the Science Museum on 26 May 1932 with Guest of Honour Miss Amelia Earhart. Edited by Chris Male MRAeS Royal Aeronautical Society www.aerosociety.com Afterburner Society News RAeS 150th ANNIVERSARY www.aerosociety.com/150 The Royal Aeronautical Society: Part 1 – The early years The Beginning “At a meeting held at Argyll Lodge, Campden Hill, Right: The first Aeronautical on 12 January 1866, His Grace The Duke of Argyll Exhibition, Crystal Palace, 1868, showing the presiding; also present Mr James Glaisher, Dr Hugh Stringfellow Triplane model W. Diamond, Mr F.H. Wenham, Mr James Wm. Butler and other exhibits. No fewer and Mr F.W. Brearey. Mr Glaisher read the following than 77 exhibits were address: collected together, including ‘The first application of the Balloon as a means of engines, lighter- and heavier- than-air models, kites and ascending into the upper regions of the plans of projected machines. atmosphere has been almost within the recollection A special Juror’s Report on on ‘Aerial locomotion and the laws by which heavy of men now living but with the exception of some the exhibits was issued. bodies impelled through air are sustained’. of the early experimenters it has scarcely occupied Below: Frederick W Brearey, Wenham’s lecture is now one of the aeronautical Secretary of the the attention of scientific men, nor has the subject of Aeronautical Society of Great classics and was the beginning of the pattern of aeronautics been properly recognised as a distinct Britain, 1866-1896. -

The Royal Society Medals and Awards
The Royal Society Medals and Awards Table of contents Overview and timeline – Page 1 Eligibility – Page 2 Medals open for nominations – Page 8 Nomination process – Page 9 Guidance notes for submitting nominations – Page 10 Enquiries – Page 20 Overview The Royal Society has a broad range of medals including the Premier Awards, subject specific awards and medals celebrating the communication and promotion of science. All of these are awarded to recognise and celebrate excellence in science. The following document provides guidance on the timeline and eligibility criteria for the awards, the nomination process and our online nomination system Flexi-Grant. Timeline • Call for nominations opens 30 November 2020 • Call for nominations closes on 15 February 2021 • Royal Society contacts suggested referees from February to March if required. • Premier Awards, Physical and Biological Committees shortlist and seek independent referees from March to May • All other Committees score and recommend winners to the Premier Awards Committee by April • Premier Awards, Physical and Biological Committees score shortlisted nominations, review recommended winners from other Committees and recommend final winners of all awards by June • Council reviews and approves winners from Committees in July • Winners announced by August Eligibility Full details of eligibility can be found in the table. Nominees cannot be members of the Royal Society Council, Premier Awards Committee, or selection Committees overseeing the medal in question. More information about the selection committees for individual medals can be found in the table below. If the award is externally funded, nominees cannot be employed by the organisation funding the medal. Self-nominations are not accepted. -

Evidence Synthesis on the EU-UK Relationship on Research and Innovation January 2018
Evidence synthesis on the EU-UK relationship on research and innovation January 2018 1. Introduction The Royal Society and the Wellcome Trust have undertaken a rapid evidence synthesis on the EU-UK research and innovation relationship as part of their Future Partnership Project. Organisations and individuals were invited to submit evidence and analyses for inclusion. Evidence was also gathered through internet searches to ensure an inclusive approach. The Annex is a summary of the methods. Two questions were used in gathering evidence and in determining the material in scope: 1. What incentives, infrastructure and mechanisms can be accessed by research and innovation organisations, funders and individuals in Member States to support collaborations? 2. How do Member States currently use and benefit from these and how might they be affected by Brexit? This paper is a synthesis of the evidence and covers funding, infrastructures, mobility, collaboration and regulation, with a focus on links between the EU and the UK. 2. Overview of the evidence base A few major reports were of particular relevance; the Royal Society’s three reports on the role of the EU in UK research and innovation and two reports commissioned from Technopolis Group by UK organisations, on the role of EU funding in UK research and innovation and the impact of collaboration: the value of UK medical research to EU science and health1,2. These documents were often referenced in other submissions. A report from the Lords Science and Technology Committee’s inquiry on EU Membership and UK Science also summarises many sources of evidence relevant to this synthesis. -
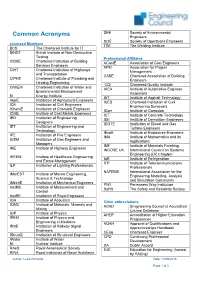
Common Acronyms Engineers SOE Society of Operations Engineers Licensed Members TWI the Welding Institute BCS the Chartered Institute for IT
SEE Society of Environmental Common Acronyms Engineers SOE Society of Operations Engineers Licensed Members TWI The Welding Institute BCS The Chartered Institute for IT BINDT British Institute of Non-Destructive Testing Professional Affiliates CIBSE Chartered Institution of Building ACostE Association of Cost Engineers Services Engineers APM Association for Project CIHT Chartered Institution of Highways Management and Transportation CABE Chartered Association of Building CIPHE Chartered Institute of Plumbing and Engineers Heating Engineering CQI Chartered Quality Institute CIWEM Chartered Institution of Water and IAEA Institute of Automotive Engineer Environmental Management Assessors EI Energy Institute IAT Institute of Asphalt Technology IAgrE Institution of Agricultural Engineers ICES Chartered Institution of Civil ICE Institution of Civil Engineers Engineering Surveyors IChemE Institution of Chemical Engineers ICorr Institute of Corrosion ICME Institute of Cast Metals Engineers ICT Institute of Concrete Technology IED Institution of Engineering IDE Institute of Demolition Engineers Designers IDGTE Institution of Diesel and Gas IET Institution of Engineering and Turbine Engineers Technology IExpE Institute of Explosives Engineers IFE Institution of Fire Engineers IMA Institute of Mathematics and its IGEM Institution of Gas Engineers and Applications Managers IMF Institute of Materials Finishing IHE Institute of Highway Engineers INCOSE UK International Council on Systems Engineering (UK Chapter) IHEEM Institute of Healthcare Engineering -

Meeting Reports
AUGUST 2014 MEETING Speaker: Andrew Conacher Topic: Sir Humphry Davy – The Davy Lamp Andrew’s talk on Sir Humphry Davy was most engaging and enjoyable. He firstly spoke on Sir Humphry’s background and life and then focused on the Davy Lamp, its context and importance. Andrew indicated that he has an ancestral family connection to Sir Humphry. The following is a brief extract from Andrew’s paper. Sir Humphry was born on 17 December 1778 in Penzance, Cornwall, England and died 29 May 1829 in Geneva, Switzerland aged 50 years. A statue stands in Penzance with him holding his safety lamp. Andrew Conacher holds up miner’s safety lamps. He was both a chemist and inventor Picture: KIRK GILMOUR, Wollongong Advertiser 6 August 2014 and was also a poet and painter. He enthusiastically turned to science and later became a professor at the Royal Institution and flame (acting as a flame arrestor) presented a series of lectures which were later published. thus preventing the methane gas burning inside the lamp to pass out He worked with a number of scientists of the day (for into the atmosphere. The lamp also example, Sir Joseph Banks, who interviewed Davy for his provided a test for the presence of role at the Royal Institution and Michael Faraday who was gases. If flammable gases were a co-worker with him) and was a popular public figure. present the flame of the lamp He made a number of scientific discoveries (for example, burnt higher and with a blue tinge. electrolysis, sodium, potassium, barium) and was knighted The lamp saved lives and helped in1812 and awarded a baronetcy in 1819. -

Wellcome Trust Annual Report and Financial Statements 2018 Is © the Wellcome Trust and Is Licensed Under Creative Commons Attribution 2.0 UK
Annual Report and Financial Statements 2018 Table of contents Report from Chair 3 Report from Director 4 Trustee’s Report 5 What we do 6 Review of Charitable Activities 7 Review of Investment Activities 18 Financial Review 28 Structure and Governance 33 Social Responsibility 38 Risk Management 40 Remuneration Report 42 Nomination Committee Report 44 Remuneration Committee Report 45 Investment Committee Report 46 Audit and Risk Committee Report 47 Independent Auditor’s Report 49 Financial Statements 59 Consolidated Statement of Financial Activities 60 Consolidated Balance Sheet 61 Statement of Financial Activities of the Trust 62 Balance Sheet of the Trust 63 Consolidated Cash Flow Statement 64 Notes to the Financial Statements 65 Reference and Administrative Details 116 2 Table of Contents Wellcome Trust Annual Report 2018 Report from Chair In March 2018, the Board of Governors and I year, driven by the performance of the investment decided to reappoint Jeremy Farrar as Director portfolio, which returned 13.4%, or 10.7% after of Wellcome for a further five years. Under his inflation. This year has seen a return of market leadership, Wellcome is ambitious, innovative and volatility as the economic cycle has matured. willing to take risks in pursuit of our mission of Central Banks, led by the US Federal Reserve, have improving health by helping great ideas to thrive. begun to raise interest rates and remove the prop to Wellcome’s work is underpinned by the financial asset prices previously provided by quantitative strength we derive from our £25.9 billion investment easing. Uncertainties about Brexit have generated portfolio. -

5. the Lives of Two Pioneering Medical Chemists.Indd
The West of England Medical Journal Vol 116 No 4 Article 2 Bristol Medico-Historical Society Proceedings The Lives of Two Pioneering Medical-Chemists in Bristol Thomas Beddoes (1760-1808) and William Herapath (1796-1868) Brian Vincent School of Chemistry, University of Bristol, Bristol, BS8 1TS. Presented at the meeting on Dec 12th 2016 ABSTRACT From the second half of the 18th century onwards the new science of chemistry took root and applications were heralded in many medical-related fields, e.g. cures for diseases such as TB, the prevention of epidemics like cholera, the application of anaesthetics and the detection of poisons in forensics. Two pioneering chemists who worked in the city were Thomas Beddoes, who founded the Pneumatic Institution in Hotwells in 1793, and William Herapath who was the first professor of chemistry and toxicology at the Bristol Medical School, located near the Infirmary, which opened in 1828. As well as their major contributions to medical-chemistry, both men played important roles in the political life of the city. INTRODUCTION The second half of the 18th century saw chemistry emerge as a fledgling science. Up till then there was little understanding of the true nature of matter. The 1 The West of England Medical Journal Vol 116 No 4 Article 2 Bristol Medico-Historical Society Proceedings classical Greek idea that matter consisted of four basic elements (earth, fire, water and air) still held sway, as did the practice of alchemy: the search for the “elixir of life” and for the “philosophers’ stone” which would turn base metals into gold. -

The Royal Society Medals and Awards Overview
The Royal Society medals and awards Overview The Royal Society has a broad range of medals including premier awards, subject specific awards and medals celebrating the communication and promotion of science. All of these work to recognise and celebrate excellence in science. The following document provides guidance in the eligibility criteria for the awards, the nomination process and online nomination system. Eligibility Awards are open to citizens of a Commonwealth country or of the Irish Republic or those who have been ordinarily resident and working in a Commonwealth country or in the Irish Republic for a minimum of three years immediately prior to being proposed. Three of our premier awards are open internationally and the Milner Award is open to European citizens and residents of 12 months or more. Full details of eligibility can be found in Appendix one. Nominees cannot be members of the Royal Society Council, Premier Awards Committee, or selection Committees. If the award is externally funded, nominees cannot be employed by the organisation funding the medal. Self-nominations are not accepted and members of the selection Committee cannot nominate for their own awards. Nominations are valid for three cycles of the award unless otherwise stated. Nominators are given the opportunity to update nominations in December each year. The full list of medals that will be open in November 2017 are: Copley Medal Royal Medals (biological, physical and interdisciplinary) Croonian Medal and Lecture Bakerian Medal and Lecture Buchanan Medal -

Royal Society, 1985
The Public Understanding of Science Report of a Royal Society adhoc Group endorsed by the Council of the Royal Society The Royal Society 6 Carlton House Terrace London SWlY 5AG CONTENTS Page Preface 5 Summary 6 1. Introduction 7 2. Why it matters 9 3. The present position 12 4. Formal education 17 5. The mass media 2 1 6. ' The scientific community 24 7. Public lectures, children's activities, museums and libraries 27 8. Industry 29 9. Conclusions and recommendations 31 Annexes A. List of those submitting evidence B. Visits and seminars C. Selected bibliography PREFACE This report was prepared by an ad hoc group under the chairmanship of Dr W.F. Bodmer, F.R.S.; it has been endorsed by the Council of the Royal Society. It deals with an issue that is important not only, or even mainly, for the scientific community but also for the nation as a whole and for each individual within it. More than ever, people need some understanding of science, whether they are involved in decision-making at a national or local level, in managing industrial companies, in skilled or semi-skilled employment, in voting as private citizens or in making a wide range of personal decisions. In publishing this report the Council hopes that it will highlight this need for an overall awareness of the nature of science and, more particularly, of the way that science and technology pervade modern life, and that it will generate both debate and decisions on how best they can be fostered. The report makes a number of recommendations. -

Wellcome Trust Annual Report and Financial Statements 2019 Is © the Wellcome Trust and Is Licensed Under Creative Commons Attribution 2.0 UK
Annual Report and Financial Statements 2019 Table of contents Report from Chair 3 Report from Director 5 Trustee’s Report 7 What we do 8 Review of Charitable Activities 9 Review of Investment Activities 21 Financial Review 31 Structure and Governance 36 Social Responsibility 40 Risk Management 42 Remuneration Report 44 Remuneration Committee Report 46 Nomination Committee Report 47 Investment Committee Report 48 Audit and Risk Committee Report 49 Independent Auditor’s Report 52 Financial Statements 61 Consolidated Statement of Financial Activities 62 Consolidated Balance Sheet 63 Statement of Financial Activities of the Trust 64 Balance Sheet of the Trust 65 Consolidated Cash Flow Statement 66 Notes to the Financial Statements 67 Alternative Performance Measures and Key Performance Indicators 114 Glossary of Terms 115 Reference and Administrative Details 116 Table of Contents Wellcome Trust Annual Report 2019 | 2 Report from Chair During my tenure at Wellcome, which ends in The macro environment is increasingly challenging, 2020, I count myself lucky to have had the which has created volatility in financial markets. opportunity to meet inspiring people from a rich Q4 2018 was a very difficult quarter, but the diversity of sectors, backgrounds, specialisms resumption of interest rate cuts by the US Federal and scientific fields. Reserve underpinned another year of gains for our portfolio. We recognise that the cycle is extended, Wellcome’s achievements belong to the people and that the portfolio is likely to face more who work here and to the people we fund – it is challenging times ahead. a partnership that continues to grow stronger, more influential and more ambitious, spurred by The team is working hard to ensure that our independence. -

Peer Review College Newsletter
Peer Review College Newsletter Winter 2014 Launch of New EPSRC Strategic plan CONTENTS Over the last five years the national and international research landscape, and the 1. Launch of New EPSRC context in which EPSRC operates, have changed and continue to develop. To take Strategic plan – page 1 account of this new environment our Strategic Plan has been up-dated, with input from our partners and communities, recognising external influences including 2. EPSRC Policy on the Use the international research landscape, global economic situation and government of Animals in Research strategies. – page 1 We have developed our goals and strategies to reflect this changing landscape and 3. College Member On-line to ensure we maintain focus on our ambitious and unwavering vision: for the UK to Training – page 3 be the best place in the world to research, discover and innovate. Our Strategic Plan also recognises the importance of working in partnership if the UK is to maintain its 4. EPSRCs Peer Review position as a world-leading location for high quality research, and be equally renowned Extranet – page 4 for its innovation. 5. Return for Amendments EPSRC’s CEO, Professor Philip Nelson said: “Our Strategic Plan describes the – page 4 potential of UK science and engineering, its importance to addressing the global and 6. The importance of Pre- domestic challenges that range from energy security to healthcare, and the vital role scores – page 5 it plays in economic growth by fuelling technological progress. It sets out how EPSRC, in partnership with the academic community and industry partners, will unlock that 7. -

1 Travis Chi Wing Lau Dissertation Abstract Prophylactic Fictions
Travis Chi Wing Lau Dissertation Abstract Prophylactic Fictions: Immunity and Biosecurity traces a prehistory for inoculation insecurity, a term I use to capture the fluid responses in literary and scientific writings to the development of inoculation as a preventative health practice. The concept of immunity, from its Roman legal origins as immunitas (i.e. exemption from civic duty), has always been a political issue regarding the role of the citizen in relation to the state. However, throughout the eighteenth and nineteenth centuries, immunity became naturalized through developments in medical theory and practice, which increasingly insisted upon the relationship among healthy nations, healthy constitutions, and healthy citizens. Arguing that the “symbolic and material linchpin” of Western modernity is a paradigm of immunity, Roberto Esposito marks in this period a pivotal shift for immunity from a passive to an active condition. Resistance to or “security” from disease became achievable by the lancet. Yet the deliberate introduction of infectious matter into the body was by no means innocuous in the eyes of the public. The stakes of what matter and how that matter was administered became, for many, a matter of life and death. Prophylactic Fictions tracks how inoculation, alongside other preventative health projects, found its way into the burgeoning apparatuses of population security aimed at mitigating and preventing mounting risks to citizens’ wellbeing. Despite the historical interrelationship between inoculation and security, only recently has scholarship in security studies and in philosophy’s “immunological turn” begun to flesh out the implications of that interrelationship. Missing from this primarily presentist scholarship are perspectives from the history of medicine and literary studies.