Volumes of Distribution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1: Clinical Pharmacokinetics 1
1: CLINICAL PHARMACOKINETICS 1 General overview: clinical pharmacokinetics, 2 Pharmacokinetics, 4 Drug clearance (CL), 6 Volume of distribution (Vd), 8 The half-life (t½), 10 Oral availability (F), 12 Protein binding (PB), 14 pH and pharmacokinetics, 16 1 Clinical pharmacokinetics General overview General overview: clinical pharmacokinetics 1 The ultimate aim of drug therapy is to achieve effi cacy without toxicity. This involves achieving a plasma concentration (Cp) within the ‘therapeutic window’, i.e. above the min- imal effective concentration (MEC), but below the minimal toxic concentration (MTC). Clinical pharmacokinetics is about all the factors that determine variability in the Cp and its time-course. The various factors are dealt with in subsequent chapters. Ideal therapeutics: effi cacy without toxicity Minimum Toxic Concentration (MTC) Ideal dosing Minimum Effective Concentration (MEC) Drug concentration Time The graph shows a continuous IV infusion at steady state, where the dose-rate is exactly appropriate for the patient’s clearance (CL). Inappropriate dosing Dosing too high in relation to the patient’s CL – toxicity likely Minimum Toxic Concentration (MTC) Minimum Effective Concentration (MEC) Dosing too low in relation to the Drug concentration patient’s CL – drug may be ineffective Time Some reasons for variation in CL Low CL High CL Normal variation Normal variation Renal impairment Increased renal blood fl ow Genetic poor metabolism Genetic hypermetabolism Liver impairment Enzyme induction Enzyme inhibition Old age/neonate 2 General overview Clinical Pharmacokinetics Pharmacokinetic factors determining ideal therapeutics If immediate effect is needed, a loading dose (LD) must be given to achieve a desired 1 concentration. The LD is determined by the volume of distribution (Vd). -

Multidrug-Resistant Gram-Negative Bacteria
Multidrug-Resistant Gram-Negative Bacteria: Trends, Risk Factors, and Treatments A worldwide public health problem, antibiotic resistance leads to treatment-resistant infections associated with prolonged hospitalizations, increased cost, and greater risk for morbidity. Lee S. Engel MD, PhD CASE INTRODUCTION An 80-year-old female nursing home resident with Antibiotics have saved the lives of millions of people a history of dementia, anemia, atrial fibrillation, and have contributed to the major gains in life ex- hypertension, incontinence, and recurrent urinary pectancy over the last century. In US hospitals, 190 tract infections (UTIs), as well as a long-term Foley million doses of antibiotics are administered each catheter, is admitted because she was found to be day.1 Furthermore, more than 133 million courses febrile and less responsive than normal. The patient of antibiotics are prescribed each year for outpatients. has no drug allergies. Upon admission, she has a However, antibiotic use has also resulted in a major temperature of 38.9ºC, heart rate of 92 beats/min, health care challenge—the development and spread and blood pressure of 106/64 mm Hg. The patient of resistant bacteria. Worldwide, antimicrobial resis- opens her eyes to stimuli but does not speak. Aside tance is most evident in diarrheal diseases, respira- from poor dentition and an irregular heart rate, tory tract infections, meningitis, sexually transmitted findings on physical examination, which includes infections, and hospital and health care–acquired in- a pulmonary and abdominal examination, are nor- fections.2 Vancomycin-resistant enterococci, methi- mal. Serum chemistries demonstrate a white blood cillin-resistant Staphylococcus aureus, multidrug-resis- cell (WBC) count of 11,300 cells/mm3. -

No Slide Title
[email protected] In Silico Drug Interaction of Long-acting # 458 Rilpivirine and Cabotegravir With Rifampin Rajith KR Rajoli1, Paul Curley1, David Back1, Charles Flexner2, Andrew Owen1, Marco Siccardi1 1 - Department of Molecular and Clinical Pharmacology, University of Liverpool, Liverpool, UK 2- Johns Hopkins University School of Medicine and Bloomberg School of Public Health, Baltimore, MD, USA Introduction Methods • Physiologically-based pharmacokinetic (PBPK) modelling represents a • Co-administration of anti-TB drugs and many antiretrovirals (ARVs) result mathematical tool to predict DDI magnitude through a detailed • 100 virtual individuals were simulated using Simbiology v.4.3.1, a in important drug-drug interactions (DDIs) understanding of mechanisms underpinning pharmacokinetics product of MATLAB (version 2013b) • Investigation of DDIs between ARVs and anti-TB drugs in individuals can • The objective of this study was to simulate the effect of rifampicin on the • PBPK models were qualified against literature data for oral formulations be complicated by numerous clinical, logistical barriers and also due to pharmacokinetics of cabotegravir and rilpivirine long-acting injectable (LAI) of rifampicin (600 mg OD, at day 6 & 14), cabotegravir and rilpivirine the risk of treatment failure intramuscular (IM) formulations using PBPK modelling (oral, single dose & steady state and IM compared to LATTE-2 studies)1-4 • Loading doses of 800 mg, 900 mg and maintenance doses of 400/800 Results mg, 600/900 mg were used for cabotegravir -

Alcohol Withdrawal
Alcohol withdrawal TERMINOLOGY CLINICAL CLARIFICATION • Alcohol withdrawal may occur after cessation or reduction of heavy and prolonged alcohol use; manifestations are characterized by autonomic hyperactivity and central nervous system excitation 1, 2 • Severe symptom manifestations (eg, seizures, delirium tremens) may develop in up to 5% of patients 3 CLASSIFICATION • Based on severity ○ Minor alcohol withdrawal syndrome 4, 5 – Manifestations occur early, within the first 48 hours after last drink or decrease in consumption 6 □ Manifestations develop about 6 hours after last drink or decrease in consumption and usually peak about 24 to 36 hours; resolution occurs in 2 to 7 days 7 if withdrawal does not progress to major alcohol withdrawal syndrome 4 – Characterized by mild autonomic hyperactivity (eg, tachycardia, hypertension, diaphoresis, hyperreflexia), mild tremor, anxiety, irritability, sleep disturbances (eg, insomnia, vivid dreams), gastrointestinal symptoms (eg, anorexia, nausea, vomiting), headache, and craving alcohol 4 ○ Major alcohol withdrawal syndrome 5, 4 – Progression and worsening of withdrawal manifestations, usually after about 24 hours from the onset of initial manifestations 4 □ Manifestations often peak around 50 hours before gradual resolution or may continue to progress to severe (complicated) withdrawal, particularly without treatment 4 – Characterized by moderate to severe autonomic hyperactivity (eg, tachycardia, hypertension, diaphoresis, hyperreflexia, fever); marked tremor; pronounced anxiety, insomnia, -
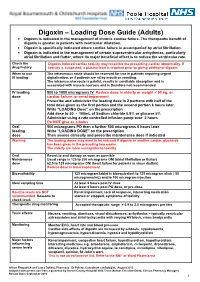
Digoxin – Loading Dose Guide (Adults) Digoxin Is Indicated in the Management of Chronic Cardiac Failure
Digoxin – Loading Dose Guide (Adults) Digoxin is indicated in the management of chronic cardiac failure. The therapeutic benefit of digoxin is greater in patients with ventricular dilatation. Digoxin is specifically indicated where cardiac failure is accompanied by atrial fibrillation. Digoxin is indicated in the management of certain supraventricular arrhythmias, particularly atrial fibrillation and flutter, where its major beneficial effect is to reduce the ventricular rate. Check the Digoxin-induced cardiac toxicity may resemble the presenting cardiac abnormality. If drug history toxicity is suspected, a plasma level is required prior to giving additional digoxin. When to use The intravenous route should be reserved for use in patients requiring urgent IV loading digitalisation, or if patients are nil by mouth or vomiting. The intramuscular route is painful, results in unreliable absorption and is associated with muscle necrosis and is therefore not recommended. IV loading 500 to 1000 micrograms IV Reduce dose in elderly or weight < 50 kg, or dose cardiac failure, or renal impairment Prescribe and administer the loading dose in 2 portions with half of the total dose given as the first portion and the second portion 6 hours later. Write “LOADING Dose” on the prescription Add dose to 50 - 100mL of Sodium chloride 0.9% or glucose 5% Administer using a rate controlled infusion pump over 2 hours Do NOT give as a bolus Oral 500 micrograms PO then a further 500 micrograms 6 hours later loading Write “LOADING DOSE” on the prescription dose Then assess clinically and prescribe maintenance dose if indicated Warning The loading doses may need to be reduced if digoxin or another cardiac glycoside has been given in the preceding two weeks. -
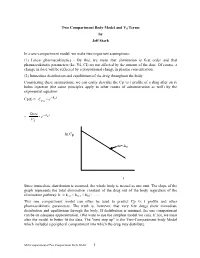
Two Compartment Body Model and Vd Terms by Jeff Stark
Two Compartment Body Model and Vd Terms by Jeff Stark In a one-compartment model, we make two important assumptions: (1) Linear pharmacokinetics - By this, we mean that elimination is first order and that pharmacokinetic parameters (ke, Vd, Cl) are not affected by the amount of the dose. Of course, a change in dose will be reflected by a proportional change in plasma concentration. (2) Immediate distribution and equilibrium of the drug throughout the body. Considering these assumptions, we can easily describe the Cp vs t profile of a drug after an iv bolus injection (the same principles apply to other routes of administration as well) by the exponential equation: − Cp(t) = Ce• kte p0 Dose − = • e kte Vd ln Cp m=-ke t Since immediate distribution is assumed, the whole body is treated as one unit. The slope of the graph represents the total elimination constant of the drug out of the body regardless of the elimination pathway: ke = kren + kmet + kbil + .…. This one compartment model can often be used to predict Cp vs t profile and other pharmacokinetic parameters. The truth is, however, that very few drugs show immediate distribution and equilibrium through the body. If distribution is minimal, the one compartment can be an adequate approximation. (We want to use the simplest model we can). If not, we must alter the model to better fit the data. The "next step up" is the Two-Compartment body Model which includes a peripheral compartment into which the drug may distribute. Multicompartmental/Two Compartment Body Model 1 THE TWO COMPARTMENT MODEL i.v. -

Pharmacokinetic Training Packet for Pharmacists
Pharmacokinetic Training Packet for Pharmacists Revised 1/09, 6/12 Original document compiled by: Elizabeth D. Hermsen, Pharm.D., M.B.A., BCPS-ID Updated by: Alan Gross, Pharm.D., BCPS Thanks to Erin Iselin, Scott McMullen, Chris Shaffer, & Keith Olsen for your thoughtful review! Any questions? Call or email Alan Gross at 559-4149/[email protected] Table of Contents Pharmacokinetic definitions and principles 3 Aminoglycoside overview 4 Extended-interval (Once daily) aminoglycoside dosing 8 Aminoglycoside pharmacokinetic calculations 10 Aminoglycoside dosing in patients with cystic fibrosis 12 Vancomycin overview and pharmacokinetic calculations 15 Clinical Pearls 21 Dialysis – Aminoglycosides and Vancomycin 21 TNMC Nephrology Protocol for Vancomycin Dosing 21 Clinical Pharmacokinetic Consult Service 23 2 Pharmacokinetic Definitions and Principles Kel, Ke, or Kd or Elimination Rate Constant 1 • The fraction or percentage of the total amount of drug in the body eliminated per unit of time. • Estimated with 2 drug levels taken between doses (the slope of the line). To be accurate, 2-4 half-lives should occur between the levels.1 -kel(τ) • In pharmacokinetic calculations, the term e represents the fraction of the serum concentration that remains. Thus, 1 - e- kel(τ) represents the fraction of the serum concentration that is eliminated. t 1/2 or Half-life 1 • The time required for the TOTAL amount of remaining drug in the body to decline by 50%. • Sometimes referred to as β t ½ to distinguish it from the distribution half-life, α t ½, used in two compartment modeling.1 Peak, C max1 • C max is the maximum measurable drug concentration at the end of an infusion BEFORE significant distribution occurs. -

Review of Pharmacokinetics and Pharmacogenetics in Atypical Long-Acting Injectable Antipsychotics
pharmaceutics Review Review of Pharmacokinetics and Pharmacogenetics in Atypical Long-Acting Injectable Antipsychotics Francisco José Toja-Camba 1,2,† , Nerea Gesto-Antelo 3,†, Olalla Maroñas 3,†, Eduardo Echarri Arrieta 4, Irene Zarra-Ferro 2,4, Miguel González-Barcia 2,4 , Enrique Bandín-Vilar 2,4 , Victor Mangas Sanjuan 2,5,6 , Fernando Facal 7,8 , Manuel Arrojo Romero 7, Angel Carracedo 3,9,10,* , Cristina Mondelo-García 2,4,* and Anxo Fernández-Ferreiro 2,4,* 1 Pharmacy Department, University Clinical Hospital of Ourense (SERGAS), Ramón Puga 52, 32005 Ourense, Spain; [email protected] 2 Clinical Pharmacology Group, Institute of Health Research (IDIS), Travesía da Choupana s/n, 15706 Santiago de Compostela, Spain; [email protected] (I.Z.-F.); [email protected] (M.G.-B.); [email protected] (E.B.-V.); [email protected] (V.M.S.) 3 Genomic Medicine Group, CIMUS, University of Santiago de Compostela, 15782 Santiago de Compostela, Spain; [email protected] (N.G.-A.); [email protected] (O.M.) 4 Pharmacy Department, University Clinical Hospital of Santiago de Compostela (SERGAS), Citation: Toja-Camba, F.J.; 15706 Santiago de Compostela, Spain; [email protected] Gesto-Antelo, N.; Maroñas, O.; 5 Department of Pharmacy and Pharmaceutical Technology and Parasitology, University of Valencia, Echarri Arrieta, E.; Zarra-Ferro, I.; 46100 Valencia, Spain González-Barcia, M.; Bandín-Vilar, E.; 6 Interuniversity Research Institute for Molecular Recognition and Technological Development, -

Practical Review of Pharm a C O L O G Y C O N C E P T S
Practical Review of Pharm a c o l o g y C o n c e p t s Sue M. Janda Nancy L. Fagan he term p h a rm a c o l o g y Pharmacology concepts are used ro u t i n e ly in nu rsing practice.T h e s e is derived from the concepts may be as simple as drug names and side effe c t s , or as G reek words “phar- c o m p l ex as pharmacokinetics or pharmacody n a m i c s . All play major makon,” meaning dru g s , roles in drug efficacy and safe t y. A practical rev i ew of pharmacology, Tand “logos,” meaning science. i n cluding pharmacokinetic and pharmacodynamic concepts, will be P h a r macology dates back to p r e s e n t e d . ancient times when man used plants and roots to treat ailments. © 2010 Society of Urologic Nurses and Associates In more recent times, the United U rologic Nurs i n g, p p . 1 5 - 2 1 . States Congress has passed re g u- lations that re q u i re drug manu- f a c t u r ers to study and pro v e Key Wo rd s : Pharmacology, adverse effects, pharmacokinetics, d r ugs are safe and eff e c t i v e pharmacodynamics, therapeutic index. b e f o r e being approved, pre- scribed, and sold to the public. O b j e c t i v e s Even with these studies, no d r ugs are perfectly safe. -

VANCOMYCIN DOSING and MONITORING GUIDELINES (NB Provincial Health Authorities Anti-Infective Stewardship Committee)
Amended: October 2020 VANCOMYCIN DOSING AND MONITORING GUIDELINES (NB Provincial Health Authorities Anti-Infective Stewardship Committee) GENERAL COMMENTS • Vancomycin is a glycopeptide antibiotic with bactericidal activity • It is active against gram-positive bacteria, including methicillin-resistant staphylococcus (MRSA) • Vancomycin is less effective than beta-lactams against Staphylococcus aureus that is susceptible to cloxacillin/methicillin • Vancomycin exhibits time-dependent killing: its effect depends primarily upon the time the concentration exceeds the organism’s Minimum Inhibitory Concentration (MIC) • These guidelines pertain to IV vancomycin only; they do not apply to PO vancomycin, which is not absorbed • Ensure that an adequate mg/kg dose and appropriate interval are ordered initially. Adjust the dose if necessary immediately; do not wait for a confirmatory trough level. • When managing a severe Staphylococcus aureus infection (e.g., bacteremia), an Infectious Diseases consultation is strongly encouraged. VANCOMYCIN IN ADULT PATIENTS ADULT INITIAL DOSE Loading dose: • Consider using a loading dose in patients with: o severe infections where rapid attainment of target level of 10-15 mg/mL is desired o significant renal dysfunction in order to decrease the time required to attain steady state • Recommended dose: 25-30 mg/kg IV o based on actual body weight, for 1 dose, followed by maintenance dose separated by recommended dosing interval o consider capping the loading dose at a maximum of 3g o loading doses DO NOT need to -

Loading Doses in Primary Care
Hull and East Riding Prescribing Committee Preventing fatalities from medication loading doses: Guidance for Primary Care Background A loading dose is an initial large dose of a medicine used to ensure a quick therapeutic response. It is usually given for a short period before therapy continues with a lower maintenance dose. The use of loading doses of medicines can be complex and error prone. Incorrect use of loading doses or subsequent maintenance regimens may lead to severe harm or death. The National Patient Safety Agency (NPSA) issued a Rapid Response Report (NPSA/2010/RRR018) in November 2010, with recommended actions aimed to reduce the number and severity of medication incidents involving incorrect prescribing or administration of loading doses and subsequent maintenance doses. These actions included production of a critical list of medicines, where incorrect loading doses or subsequent maintenance doses are likely to cause harm and ensuring healthcare professionals in primary care are aware of when to challenge abnormal doses of medicines on the agreed critical list. This guidance has been developed as a response to these recommended actions. Critical List Agreed critical list of drugs most likely to cause harm as a result of incorrect prescribing or administration of loading dose or subsequent maintenance dose are: Amiodarone Digoxin Phenytoin Warfarin Best practice in the prescribing, supply and administration of drugs requiring loading dose 1. Where a loading dose is prescribed or recommended, ensure that details of on- going treatment and titration to maintenance dose are clear, and in line with national or local guidelines. 2. Challenge any abnormal prescribing or treatment recommendations (see page 2). -

These Highlights Do Not Include All the Information Needed to Use ACETYLCYSTEINE INJECTION Safely and Effectively
ACETYLCYSTEINE- acetylcysteine injection, solution Fresenius Kabi USA, LLC ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ACETYLCYSTEINE INJECTION safely and effectively. See full prescribing information for ACETYLCYSTEINE INJECTION. ACETYLCYSTEINE injection Initial U.S. Approval: 2004. INDICATIONS AND USAGE Acetylcysteine Injection is an antidote for acetaminophen overdose indicated to prevent or lessen hepatic injury after ingestion of a potentially hepatotoxic quantity of acetaminophen (1) DOSAGE AND ADMINISTRATION The total dose of Acetylcysteine Injection is 300 mg/kg given as 3 separate doses and administered over a total of 21 hrs. Please refer to the guidelines below for dose preparation based upon patient weight. Dosing for patients who weigh 5 kg to 20 kg ( 2.1): Loading Dose: 150 mg/kg diluted in 3 mL/kg of diluent* administered over 1 hr Second Dose: 50 mg/kg diluted in 7 mL/kg of diluent* administered over 4 hrs Third Dose: 100 mg/kg diluted in 14 mL/kg of diluent* administered over 16 hrs Dosing for patients who weigh 21 kg to 40 kg ( 2.1): Loading Dose: 150 mg/kg diluted in 100 mL of diluent* administered over 1 hr Second Dose: 50 mg/kg diluted in 250 mL of diluent* administered over 4 hrs Third Dose: 100 mg/kg diluted in 500 mL of diluent* administered over 16 hrs Dosing for patients who weigh 41 kg to 100 kg ( 2.1): Loading Dose: 150 mg/kg diluted in 200 mL of diluent* administered over 1 hr Second Dose: 50 mg/kg diluted in 500mL of diluent* administered over 4 hrs Third Dose: 100 mg/kg diluted in 1,000 mL of diluent* administered over 16 hrs * Acetylcysteine Injection is compatible with the following diluents; 5% Dextrose in Water, 0.45% Sodium Chloride Injection, and Sterile Water for Injection Dosing for patients who weigh more than 100 kg ( 2.1): No specific studies have been conducted to evaluate the use of or necessity of dosing adjustments in patients weighing over 100 kg.