February 12, 2015 Commissioner Vytenis Andriukaitis European
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Page 1 of 15 Mr Jean-Claude Juncker President European Commission Cc
Mr Jean-Claude Juncker President European Commission cc: Frans Timmermans, First Vice-President, in charge of Better Regulation, Inter-Institutional Relations, the Rule of Law and the Charter of Fundamental Rights Andrus Ansip, Vice-President for the Digital Single Market Jyrki Katainen, Vice-President for Jobs, Growth, Investment and Competitiveness Maroš Šefčovič, Vice-President for the Energy Union Vytenis Andriukaitis, Commissioner for Health and Food Safety Elžbieta Bieńkowska, Commissioner for Internal Market, Industry, Entrepreneurship and SMEs Violeta Bulc, Commissioner for Transport Miguel Arias Cañete, Commissioner for Climate Action and Energy Corina Creţu, Commissioner for Regional Policy Carlos Moedas, Commissioner for Research, Science and Innovation Cecilia Malmström, Commissioner for Trade Pierre Moscovici, Commissioner for Economic and Financial Affairs, Taxation and Customs Tibor Navracsics, Commissioner for Education, Culture, Youth and Sport Günther Öttinger, Commissioner for Budget and Human Resources Marianne Thyssen, Commissioner for Employment, Social Affairs, Skills and Labour Mobility Karmenu Vella, Commissioner for Environment, Maritime Affairs and Fisheries Margrethe Vestager, Commissioner for Competition Brussels, 16 June 2017 Re: Contribute to economic growth and climate change mitigation through a EU Cycling Strategy Dear President Juncker, With this letter, signed by leaders from businesses, public authorities and civil society, we call upon the European Commission to unlock the potential for creating jobs -

THE LITHUANIAN WORLD-WIDE DAILY Novambar 12
3106 p GRATIS ? n REFERENS^F C^ESS "A3HIN6T0N OC 20025 UŽSIENIO LIETUVIŲ DIENRAŠTIS 4545 WEST 63rd STREET • CHICAGO, ILLINOIS 60629 TEL: 773-585-9500 • FAX: 773-585-8284 • DRAUGAS@EARTHUNICNET PERtOCHCALS THE LITHUANIAN WORLD-WIDE DAILY Novambar 12 . 1996 Nr. 221 VoL LXXXVI Kaina 50 c. ANTRADIENIS - TUESDAY, LAPKRITIS - NOVEMBER 12, 1996 Kas tarnaus Lietuvai Pasaulio naujienos ateinančius 4 metus? (Remiantis DPA, Reuter, BNS INTERFAX, ITAR-TASS, BelaPAN žinių agentūrų pranešimais) Vilnium, lapkričio 11 d. (BNS) Žvaliauskas, Vaclovas Lapė, - Rinkimų komisijai paskelbus Rytas Kupčinskas, Vladimiras Vašingtonas. Jungtinių Santiago. Lotynų Amerikos urminius rezultatus, galutinai Jarmolenko, Simas Petrikis, Valstijų Senato respublikonų valstybių bei Ispanijos ir Portu >aaiškėjo, kad didžiausią frak- Gražina Imbrasienė, Petras vadovas Trent Lott šeštadienį galijos valdovų susitikime Ku nją būsimajame Seime sudarys Šakalinis, Stasys Malkevičius, pažadėjo bendradarbiauti su an bos prezidentas Fidel Castro konservatorių frakcija, ture- Juozas Dringelis, Žibartas trai kadencijai perrinku JAV užsipuolė Jungtines Valstijas, lianti 70 narių. Tai nesudaro Jackūnas, Nijolė Ambrazaitytė, prezidentu Bill Clinton, jei jis kad šios, kišdamosis į Lotynų absoliučios daugumos 141 vietą Stasys Stačiokas, Algimantas nuoširdžiai sieks subalansuoti Amerikos valstybių reikalus, turinčiame Seime. Tačiau kon Sėjūnas, Juozas Galdikas, šalies biudžetą ir sumažinti vy žlugdo jų kultūrą. „Mūsų kul servatoriai tikrai turės daugu Vytautas Knašys, Vincas Gir riausybę. Lott šeštadienį pareiš tūrą žlugdo šio hegemono kont mą pavasarį, kai bus surengti nius, Romualdas Sikorskis, kė, jog respublikonai yra nusi roliuojamos visuomenės infor nauji rinkimai 4 apygardose, Alfredas Stasiulevičius, Al teikę užmiršti rinkimų kampa mavimo priemonės, tuo tarpu, kur jie neįvyko. girdas Petruševičius, Vladas nijos kartėlį ir pradėti dirbti kai ta pati valstybė organizuo kartu šalies labui. -

THE JUNCKER COMMISSION: an Early Assessment
THE JUNCKER COMMISSION: An Early Assessment John Peterson University of Edinburgh Paper prepared for the 14th Biennial Conference of the EU Studies Association, Boston, 5-7th February 2015 DRAFT: Not for citation without permission Comments welcome [email protected] Abstract This paper offers an early evaluation of the European Commission under the Presidency of Jean-Claude Juncker, following his contested appointment as the so-called Spitzencandidat of the centre-right after the 2014 European Parliament (EP) election. It confronts questions including: What will effect will the manner of Juncker’s appointment have on the perceived legitimacy of the Commission? Will Juncker claim that the strength his mandate gives him license to run a highly Presidential, centralised Commission along the lines of his predecessor, José Manuel Barroso? Will Juncker continue to seek a modest and supportive role for the Commission (as Barroso did), or will his Commission embrace more ambitious new projects or seek to re-energise old ones? What effect will British opposition to Juncker’s appointment have on the United Kingdom’s efforts to renegotiate its status in the EU? The paper draws on a round of interviews with senior Commission officials conducted in early 2015 to try to identify patterns of both continuity and change in the Commission. Its central aim is to assess the meaning of answers to the questions posed above both for the Commission and EU as a whole in the remainder of the decade. What follows is the proverbial ‘thought piece’: an analysis that seeks to provoke debate and pose the right questions about its subject, as opposed to one that offers many answers. -

Mr Vytenis ANDRIUKAITIS European Commissioner for Health European Commission Rue De La Loi, 200 1049 Brussels
Mr Vytenis ANDRIUKAITIS European Commissioner for Health European Commission Rue de la Loi, 200 1049 Brussels Ref: RB.RT.mcf/67.190 29 June 2015 Dear Commissioner, Following the meeting that my EFPIA colleagues and I had on 28 May 2015 with members of your cabinet in relation to the ongoing crisis in Greece, I am writing to follow up with more details on our concerns about what might happen within the medicines market in the event that Greece fails to reach a deal with its creditors and is forced to leave the Euro. In the worst-case scenario of ‘Grexit’, we believe the integrity of the medicines supply chain may be in jeopardy, which would create a risk to public health. As such we believe it prudent and responsible to ensure there is a dialogue between the Commission and the pharmaceutical industry on concrete contingency plans. There are two broad categories of issues that we believe may disrupt the supply of medicines under the Grexit scenario: 1. The first relates to the potential for general practical disruption in the operation of the business environment. Technical breakdown in infrastructure supporting transactions, uncertainty on the validity of contracts, coupled with general social unrest, is likely to lead to problems throughout the economy. The Greek medicines supply chain is complicated compared with other European member states and therefore we think that the medicines supply chain may be particularly vulnerable to the sort of disruption described here. We believe, for example, that several hundred wholesalers are involved in the purchase and distribution of medicines to Greek pharmacies (a supply chain that is much more fragmented than in other EU countries). -
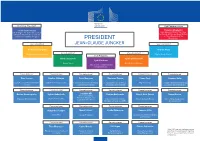
President High Representative
First Vice-President High Representative Frans Timmermans Federica Mogherini Better Regulation, Inter-Institutional High Representative of the Union Relations, the Rule of Law and the for Foreign Affairs and Security Poli- Charter of Fundamental Rights cy / Vice-President of the PRESIDENT Commission Vice-President JEAN-CLAUDE JUNCKER Vice-President Kristalina Georgieva Andrus Ansip Vice-President Vice-President Budget & Human Resources Digital Single Market Vice-President Alenka Bratušek Valdis Dombrovskis Jyrki Katainen Energy Union Euro & Social Dialogue Jobs, Growth, Investment and Competitiveness Commissioner Commissioner Commissioner Commissioner Commissioner Commissioner Vĕra Jourová Günther Oettinger Pierre Moscovici Marianne Thyssen Corina Creţu Johannes Hahn Justice, Consumers and Gender Digital Economy & Society Economic and Financial Affairs, Employment, Social Affairs, Regional Policy European Neighbourhood Policy Equality Taxation and Customs Skills and Labour Mobility & Enlargement Negotiations Commissioner Commissioner Commissioner Commissioner Commissioner Commissioner Dimitris Avramopoulos Vytenis Andriukaitis Jonathan Hill Elżbieta Bieńkowska Miguel Arias Cañete Neven Mimica Financial Stability, Financial Services and Health & Food Safety Migration & Home Affairs Capital Markets Union Internal Market, Industry, Climate Action & Energy International Cooperation Entrepreneurship and SMEs & Development Commissioner Commissioner Commissioner Commissioner Margrethe Vestager Maroš Šefčovič Cecilia Malmström Karmenu Vella Competition Transport & Space Trade Environment, Maritime Affairs and Fisheries Commissioner Commissioner Commissioner Commissioner Tibor Navracsics Carlos Moedas Phil Hogan Christos Stylianides * The HRVP may ask the Commissioner Education, Culture, Youth and Research, Science Agriculture & Humanitarian Aid & (and other commissioners) to deputise Citizenship and Innovation Rural Development Crisis Management for her in areas related to Commission competence. -

Vytenis Andriukaitis
VYTENIS ANDRIUKAITIS Personal details • Lithuanian • Born 9 August 1951, in Yakutsk Autonomous Republic, Russia (USSR) • Married with three children Education 1979 - 1984: Vilnius University, Faculty of History (Master’s degree and lecturer in history and political science) 1969 - 1975: Kaunas Institute of Medicine (medical doctor) Political career • 2012 - 2014: Minister of Health, Republic of Lithuania • 2014: Vice-President of the 67th World Health Assembly • 1992 - 2014: Member of Seimas, Parliament of the Republic of Lithuania, 6 consecutive terms • 2008 – 2012: Member, COSAC (Conference of Parliamentary Committees for Union Affairs of Parliaments of the European Union) • 2004 – 2008: Director, Institute of Social and Economic Studies • 2002 – 2003: Leader of the Lithuanian delegation to the Convention on the Future of Europe • 2001 – 2004: Deputy Speaker of the Seimas • 2001 – 2004: Chair, EU Affairs Committee of the Seimas • 2001 – 2002: Chair, Baltic Assembly • 2000 – 2004: Member of the PES Presidium and Council • 1999 – 2000: Chair, Lithuanian Social Democratic Party • 1994 – 1996: Member, Parliamentary Assembly of the Council of Europe’s Legal Affairs and Human Rights Committee • 1990 - 1992: Signatory to Act of Independence of the Republic of Lithuania and co-author of its Constitution • 1989 Founder of Social Democratic Party of Lithuania • 1969 – 1991: Actively involved in the anti-Soviet movement Medical career • 1975 – 1976: Medical doctor, Kaunas County Hospital • 1976 – 1984: Surgeon, Ignalina Central Hospital • 1985 – 1993: Cardio-surgeon, Vilnius Republic Clinical Hospital Awards: WHO World No Tobacco Day Award (2014), Commander’s Grand Cross of the Order of Lithuanian Grand Duke Gediminas (2004), Ordem do Mérito Grã-Cruz of the Portuguese Republic (2003) Publications: various legislative acts and amendments, contributions to the Convention on the Future of Europe, books and press articles on the history of medicine, surgery, political science and labor market organization. -

Jav Senatas Ratifikavo Nato Sutartį
SECOND CLASS USPS 157-580 — THE LITHUANIAN NATIONAL NEWSPAPER 19807 CHEROKEE AVENUE CLEVELAND, OHIO 44119 VOL. LXXXVIII 2003 MAY - GEGUŽĖS 13, Nr. 19 DIRVA LIETUVIŲ TAUTINĖS MINTIES LAIKRAŠTIS i JAV SENATAS RATIFIKAVO i NATO SUTARTĮ Vašingtonas, gegužės 8 d. (ELTA). JAV Senato daugumos vadas Bill Frist tvirtina, kad Jungtinėse Valstijose NATO plėtra yra klausimas be opozicijos. Šį senatoriaus respublikono teiginį ketvirtadienį akivaizdžiai pademonstravo septynių pakviestų į Aljansą valstybių, tarp jų ir Lietuvos, prisijungimo protokolų ratifikavimo procedūra Senate. Iš 100 JAV Senato narių NATO plėtrai pritarė visi 96 senatoriai, dalyvavę posėdyje. “Aš jau seniai nemačiau tokio vieningo Senato ir tokios vieningos tautos”, - sakė Senato posėdyje B. Frist. Senato daugumos vadovas sveikino pakviestas į Aljansą valstybes Bulgariją, Estiją, Latviją, Lietuvą, Rumuniją, Slovakiją ir Slovėniją už ryžtą, siekiant tikslo, už paramą išlaisvinant Iraką. Senato mažumos vadas demokratas Tom Daschle pažymėjo, kad NATO plėtra - partnerystės, garantuosiančios pastovumą pasaulyje, pradžia. JAV Senato rūmuose ratifikavimo procedūrą stebėjo ir visų septynių praėjusių metų lapkritį Prahoje į Aljansą pakviestų valstybių delegacijos. Lietuvos delegacijai Vašingtone JAV senatorius George V. Voinovich (R-OH) ir Janet Voinovich sveikina LR ambasadorių Vygaudą vadovauja užsienio reikalų ministras Antanas Valionis. Ušacką, kai Senatas vienbalsiai ratifikavo Lietuvos ir kitų valstybių sutartis būti NATO narėmis. Po sutarties ratifikavimo ketvirtadienį surengtas į NATO Vidury LR Garbės konsule Ingrida Bublienė. Sen. Voinovich Press Office nuotr. pakviestų valstybių užsienio reikalų ministrų susitikimas su JAV prezidentu George W. Bush, viceprezidentu Richard Cheney, Valstybės sekretoriumi Colin Powell, Gynybos sekretoriumi PASIRAŠYTAS LIETUVOS IR RUSIJOS READMISIJOS SUSITARIMAS Donald Rumsfeld. V iln iu s, gegužės 8 d. vos ir Rusijos konsultacijos, misijos bei ratifikuoti valsty Baltųjų rūmų Rožių sodelyje pakviestųjų į NATO valstybių (ELTA). -

EU-27 WATCH No.7
EU-27 WATCH No. 7 ISSN 1610-6458 Issued in September 2008 Edited by the Institute for European Politics (IEP), Berlin in collaboration with the Austrian Institute of International Affairs, Vienna Institute for International Relations, Zagreb Bulgarian European Community Studies Association, Institute for World Economics of the Hungarian Sofia Academy of Sciences, Budapest Center for European Studies / Middle East Technical Institute for Strategic and International Studies, University, Ankara Lisbon Centre européen de Sciences Po, Paris Institute of International and European Affairs, Centre d’étude de la vie politique, Université libre de Dublin Bruxelles Institute of International Relations, Prague Centre d’Etudes et de Recherches Européennes Institute of International Relations and Political Robert Schuman, Luxembourg Science, Vilnius University Centre of International Relations, Ljubljana Istituto Affari Internazionali, Rome Cyprus Institute for Mediterranean, European and Latvian Institute of International Affairs, International Studies, Nicosia Riga Danish Institute for International Studies, Mediterranean Academy of Diplomatic Studies, Copenhagen University of Malta Elcano Royal Institute and UNED University, Madrid Netherlands Institute of International Relations European Institute of Romania, Bucharest ‘Clingendael’, The Hague Federal Trust for Education and Research, London Slovak Foreign Policy Association, Bratislava Finnish Institute of International Affairs, Helsinki Stockholm International Peace Research Institute Foundation -

World AIDS Day 2015: Joint Statement by HR/VP Mogherini And
European Commission - Statement World AIDS Day 2015: Joint Statement by HR/VP Mogherini and Commissioners Andriukaitis, Mimica and Moedas Brussels, 30 November 2015 On the eve of World AIDS Day, High Representative of the Union for Foreign Affairs and Security Policy and Vice-President of the Commission, Federica Mogherini, Commissioner for Health & Food Safety, Vytenis Andriukaitis, Commissioner for International Cooperation and Development, Neven Mimica and Commissioner for Research, Science and Innovation, Carlos Moedas, on behalf of the Commission, expressed their commitment to maintaining momentum in the global fight against HIV/AIDS and their determination to achieve the global target of ending AIDS by 2030. "No one facing AIDS should be left behind. Worldwide, there are still 2 million people diagnosed with HIV each year, with 1.4 million of them in sub-Saharan Africa which is the region most affected by the disease. Today, a total of 36.9 million people are living with HIV/AIDS. And it is with consternation that we are witnessing the highest number of new HIV infections in 2014 in Europe. However, much progress has been made. New infections have decreased by 35% since 2000. A decrease of 42% has been recorded for AIDS-related deaths since the peak in 2004. 15.8 million people living with HIV are now accessing life-saving treatment. The world has exceeded the AIDS targets of Millennium Development Goal (MDG) 6, halting and reversing the spread of HIV, and is looking to end the AIDS epidemic by 2030 as part of the Sustainable Development Goals (SDGs) that were adopted by the UN this year. -

Vilniaus Regiono Vystymo Programos Projektas
I...II..1 367 P6 GRATI3 THE LIBRARY ©F CONORESS EUROPEAN READING ROOM Serial» Division Wa«hington Dc 20540-4830 NEVVSPAPER - DO NOT DELAY - Date Mailed 06-27-02 UŽSIENIO LIETUVIŲ DIENRAŠTIS 4545 VVEST 63rd STREET • CHICAGO, ILLINOIS 60629 PERIODICALS THE LITHUANIAN WORLD-WIDE DAILY TEL.: 773-585-9500 • FAX: 773-585-8284 • WWW.DRAUGAS.ORG June 28, 2002 PENKTADIENIS - FRIDAY, BIRŽELIO- JUNE 28, 2002 Nr 125 Vol. LXXXX Kaina 50 c. Šiame numeryje: Vilniaus regiono vystymo programos projektas Lenkijos visuomeninė orga gramą, parašytą netaisyklinga nomistų teismui, remia vien programą? Ar Lietuvoje nėra mis Pultuske (Lenkija) įvyko nuomone, Projekto įgyvendini 52-ųjų Čikagos nizacija „Wspolnota Polska”, lietuvių kalba. Primename, lenkiškas mokyklas. valstybinių institucijų, geban Lietuvos ir Lenkijos delegacijų mas pagyvintų Lietuvos ir žaidynių plaukikų kurios tikslas — rūpintis už kad organizacijos vadovas J. Kodėl Lietuvai priešiškai čių parengti Rytų Lietuvos susitikimas, skirtas Vilniaus Lenkijos bendradarbiavimą, Stelmachovskis prieštaravo nusiteikusi organizacija kuria programą? regiono vystymo programos paspartintų regiono ekonomi pasiekti rezultatai; sienio lenkais, parengė plačią Vilniaus regiono vystymo pro- Šalčininkų rajono lenkų auto- Vilniaus regiono vystymo 2001 m. vasario 13-15 dieno- projektui aptarti. Dalyvių nę plėtrą. Nukelta į 3 psl. dar apie III Tautinę olimpiadą; susipažinkime Konservatoriai Seimas teigiamai įvertino su broliais Paškais. įžvelgia sąmokslą 2 psl. prieš V. Landsbergį derybų su Europos Sąjunga eigą Opozicinę Tėvynės sąjungą Vilnius, birželio 27 d. (BNS) rinti iki stojimo į ES politinį Apie 2025 m. pusė (Lietuvos konservatorius) pa — Seimas ketvirtadienį pri pastovumą valstybėje. pasaulio gyventojų piktino, jų teigimu, „nesoli- ėmė nutarimą, kuriame teigia „Turėtume padaryti viską, mai įvertino derybų dėl stoji kad vidinė situacija Lietuvoje susikalbės angliškai. -

Open Letter to the European Commission – EU Initiatives and Progress on Chronic Diseases
OPEN LETTER Mr Frans Timmermans, European Commission First Vice-President Mr Vytenis Andriukaitis, European Commissioner for Health and Food Safety Mr Jyrki Katainen, European Commission Vice-President for Jobs, Growth, Investment & Competitiveness Brussels, 08 July 2016 Dear First-Vice President Timmermans, Dear Commissioner Andriukaitis, Dear Vice-President Katainen, Subject: EU Initiatives and Progress on Chronic Diseases We, the undersigned health organisations, would like to express our appreciation to the European Commission for organising the stakeholder conference ‘Towards better prevention and management of chronic diseases’ on 21 April. However, it struck some attendees at the conference that no new proposals were put forward by the Commission. Apart from specific projects or activities with limited scope, we felt there was no commitment by the Commission to act on chronic diseases (CDs) in a really comprehensive approach. Surely, in the light of the economic burden that CDs place on Member States, the European Commission can do much better and, in the words of the Austrian health attaché, ‘aim for the big cake rather than the cookies’. OPEN LETTER | EU INITIATIVES AND PROGRESS ON CHRONIC DISEASES 1 We are already facing a very serious situation, as chronic diseases impose a significant cost (human suffering, reduced workforce, social exclusion, health inequalities etc.) and put a tremendous financial burden on health-care systems. Seventy to eighty per cent of health-care costs are spent on chronic diseases. This corresponds to €700 billion per annum in the European Union and the figures are expected to rise in the coming years; the situation will not improve if we do not act now. -

Lietuvos Respublikos Konstitucijos Dvidešimtmetis: Patirtis Ir Iššūkiai
LIETUVOS RESPUBLIKOS KONSTITUCIJOS dvidešimTMETIS: PATIRTIS IR iššūkiai RECENZUOtų mokslinių STRaipsnių RINKINYS 1992 m. spalio 25 d. Vilniečiai balsuoja per LR Seimo rinkimus ir referendume dėl LR Konstitucijos. Fotografas Viktoras Kapočius. Lietuvos centrinis valstybės archyvas. 1992 m. LR Konstitucijos projekto derinimo grupė. Stovi: Vytautas Sinkevičius, Narcizas Rasimavičius, Aurimas Taurantas, Juozas Žilys, Egidijus Jarašiūnas, Šarūnas Adomavičius, Vytenis Andriukaitis. Sėdi: Petras Giniotas, Romualdas Ozolas, Vytautas Landsbergis, Eduardas Vilkas, Kęstutis Lapinskas. Fotografas Zenonas Nekrošius. LR Konstitucijos ir Lietuvos Respublikos įstatymo „Dėl Lietuvos Respublikos Konstitucijos įsigaliojimo tvarkos“ pasirašymo ceremonija Aukščiausiosios Tarybos-Atkuriamojo Seimo Prezidiumo posėdžių salėje (dabar Konstitucijos salė). Vilnius, 1992 m. lapkričio 6 d. Fotografas Jonas Juknevičius. Lietuvos centrinis valstybės archyvas. LIETUVOS RESPUBLIKOS KONSTITUCIJOS dvidešimTMETIS: PATIRTIS IR iššūkiai RECENZUOtų mokslinių STRaipsnių RINKINYS VILNIAUS UNIVERSITETO Teisės FAKULTETO Viešosios teisės KATEDRA LIETUVOS NOTARų RūmAI UDK 342.5(474.5) Li317 Leidinį SUDARė: Prof. dr. (HP) Egidijus Kūris ir dr. Elena Masnevaitė Mokslinis redaktorius prof. dr. (HP) Egidijus Kūris RECENZENTAI: Prof. dr. Armanas ABRAMAVIČIUS – Vilniaus universiteto Teisės fakulteto Baudžiamosios justicijos katedra Prof. dr. Arvydas ANDRUŠKEVIČIUS – Vilniaus universiteto Teisės fakulteto Viešosios teisės katedra Prof. dr. Saulius KATUOKA – Mykolo Romerio universiteto Teisės fakulteto Tarptautinės ir Europos Sąjungos teisės katedra Kalbos redaktorė Edita Birulienė Viršelyje panaudota Gedimino Bartuškos (ELTA) nuotr. Straipsnių rinkinys išleistas Lietuvos notarų rūmų iniciatyva ir lėšomis 2012 m. gruodžio 1 d. minimos Lietuvos notariato reformos 20-osios metinės www.notarai.lt Tiražas 600 egz. Spausdino UAB „Druka“ Mainų g. 5, LT-94101 Klaipėda El. p. [email protected] www.druka.lt © Lietuvos notarų rūmai ir straipsnių autoriai, 2012 ISBN 978-609-404-133-4 TURINYS įŽANGOS ŽODIS 7 Jevgenij Machovenko .