Characterizing the Influence of Gap Junction Communication on Cell Volume Dynamics Bona Mu Buffalo State College, [email protected]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
ION CHANNEL DIVERSITY and CHARACTERIZATION
ION CHANNEL DIVERSITY and CHARACTERIZATION Voltage clamp techniques K channels Na channels Ca channels Ligand-gated channels Channelopathies OUTLINE Voltage clamp techniques whole cell, single channel, gating K channels Na channels Ca channels Cardiac AP Na nerve vs cardiac Ica: L vs T type; drugs (BayK, nitrendipine) Ito: inactivation, subtypes Kv1.4, Kv4.2/3, accessory subunits Ikr,Iks, Ikur (drugs dofetilide) IK1 – rectification Cardiac channelopathies (LQTS, SQTS, Brugada syndrome) Ligand-gated channels (AChR) Pancreatic beta cell channels (KATP, ICa) Voltage clamp techniques Capacitance currents (Ic) and ionic currents (Ii) are activated by rapid changes in membrane potential using voltage clamp Variable Vtest can be applied with voltage clamp 2.0 sec 40 duration 20 (10 sec between each pulse) 0 -20 mV test -40 V -60 -80 Simplified schematic of voltage clamp circuit Original patch clamp recordings (1981) Pflugers Arch 391: 85-100 Four modes of patch clamp technique High-throughput, automated patch clamp instruments EVOLUTION and Ion channel diversity Diversity of ion channels Example: nematode C. elegans 73 K channels (20 6 TM, 3 IRK, 50 TWIK) 89 ligand-gated channels (42 ACh, 37 inhibitory GABAA or glutamate, 10 excitatory glutamate) 5 voltage-gated Ca channels 6 chloride channels 24 gap junction channels (connexins) 22 mechanosensitive channels 6 cyclic-nucleotide gated channels 11 TRP-related channels Total: 236 channel subunit genes Origin of ion channel diversity 1) gene duplication & divergence 2) alternative mRNA splicing 3) -

Electrical Synapses Are Drivers of Neural Plasticity Through Passage of Small Molecules
Electrical Synapses are Drivers of Neural Plasticity Through Passage of Small Molecules Lisa Voelker A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2019 Reading Committee: Jihong Bai, Chair Linda Buck Cecilia Moens Program Authorized to Offer Degree: Molecular and Cellular Biology ©Copyright 2019 Lisa Voelker 2 University of Washington Abstract Electrical Synapses are Drivers of Neural Plasticity through Passage of Small Molecules Lisa Voelker Chair of the Supervisory Committee: Jihong Bai Department of Biochemistry In order to respond to changing environments and fluctuations in internal states, animals adjust their behavior through diverse neuromodulatory mechanisms. In this study we show that electrical synapses between the ASH primary quinine-detecting sensory neurons and the neighboring ASK neurons are required for modulating the aversive response to the bitter tastant quinine in C. elegans. Mutant worms that lack the electrical synapse proteins INX-18 and INX-19 become hypersensitive to dilute quinine. Cell-specific rescue experiments indicate that inx-18 operates in ASK while inx-19 is required in both ASK and ASH for proper quinine sensitivity. Imaging analyses find that INX-19 in ASK and ASH localizes to the same regions in the nerve ring, suggesting that both sides of ASK-ASH electrical synapses contain INX-19. While inx-18 and inx-19 mutant animals have a similar behavioral phenotype, several lines of evidence suggest the proteins encoded by these genes play different roles in modulating the aversive quinine response. First, INX-18 and INX-19 localize 3 to different regions of the nerve ring, indicating that they are not present in the same synapses. -
New Group of Transmembrane Proteins Associated with Desiccation Tolerance in the Anhydrobiotic Midge Polypedilum Vanderplanki Taisiya A
www.nature.com/scientificreports OPEN New group of transmembrane proteins associated with desiccation tolerance in the anhydrobiotic midge Polypedilum vanderplanki Taisiya A. Voronina1,6, Alexander A. Nesmelov1,6, Sabina A. Kondratyeva1, Ruslan M. Deviatiiarov1, Yugo Miyata2, Shoko Tokumoto3, Richard Cornette2, Oleg A. Gusev1,4,5, Takahiro Kikawada2,3* & Elena I. Shagimardanova1* Larvae of the sleeping chironomid Polypedilum vanderplanki are known for their extraordinary ability to survive complete desiccation in an ametabolic state called “anhydrobiosis”. The unique feature of P. vanderplanki genome is the presence of expanded gene clusters associated with anhydrobiosis. While several such clusters represent orthologues of known genes, there is a distinct set of genes unique for P. vanderplanki. These include Lea-Island-Located (LIL) genes with no known orthologues except two of LEA genes of P. vanderplanki, PvLea1 and PvLea3. However, PvLIL proteins lack typical features of LEA such as the state of intrinsic disorder, hydrophilicity and characteristic LEA_4 motif. They possess four to fve transmembrane domains each and we confrmed membrane targeting for three PvLILs. Conserved amino acids in PvLIL are located in transmembrane domains or nearby. PvLEA1 and PvLEA3 proteins are chimeras combining LEA-like parts and transmembrane domains, shared with PvLIL proteins. We have found that PvLil genes are highly upregulated during anhydrobiosis induction both in larvae of P. vanderplanki and P. vanderplanki-derived cultured cell line, Pv11. Thus, PvLil are a new intriguing group of genes that are likely to be associated with anhydrobiosis due to their common origin with some LEA genes and their induction during anhydrobiosis. Anhydrobiosis is the ability of an organism to survive complete desiccation in the ametabolic state. -

Src Regulation of Cx43 Phosphorylation and Gap Junction Turnover
biomolecules Article Src Regulation of Cx43 Phosphorylation and Gap Junction Turnover Joell L. Solan 1 and Paul D. Lampe 1,2,* 1 Translational Research Program, Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA; [email protected] 2 Department of Global Health, Pathobiology Program, University of Washington, Seattle, WA 98109, USA * Correspondence: [email protected] Received: 27 October 2020; Accepted: 22 November 2020; Published: 24 November 2020 Abstract: The gap junction protein Connexin43 (Cx43) is highly regulated by phosphorylation at over a dozen sites by probably at least as many kinases. This Cx43 “kinome” plays an important role in gap junction assembly and turnover. We sought to gain a better understanding of the interrelationship of these phosphorylation events particularly related to src activation and Cx43 turnover. Using state-of-the-art live imaging methods, specific inhibitors and many phosphorylation-status specific antibodies, we found phospho-specific domains in gap junction plaques and show evidence that multiple pathways of disassembly exist and can be regulated at the cellular and subcellular level. We found Src activation promotes formation of connexisomes (internalized gap junctions) in a process involving ERK-mediated phosphorylation of S279/282. Proteasome inhibition dramatically and rapidly restored gap junctions in the presence of Src and led to dramatic changes in the Cx43 phospho-profile including to increased Y247, Y265, S279/282, S365, and S373 phosphorylation. Lysosomal inhibition, on the other hand, nearly eliminated phosphorylation on Y247 and Y265 and reduced S368 and S373 while increasing S279/282 phosphorylation levels. We present a model of gap junction disassembly where multiple modes of disassembly are regulated by phosphorylation and can have differential effects on cellular signaling. -
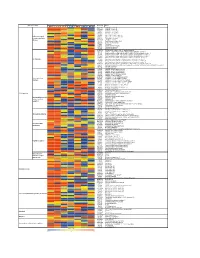
Supporting Figure 2
Normalized value Functional class Genbank Name Naive SC 1h SC 6h SC 24h WM 1h WM 6h WM 24h AB016161 GABA-B receptor 1d AF109405 GABA-B receptor 2a M35077 Dopamine-1A receptor S46131 Dopamine-1A receptor M84009 Dopamine receptor D4 U13368 Adrenergic receptor, alpha 1a G Protein-coupled M60654 Adrenergic receptor, alpha 1d receptors and their M64236 Tachykinin 1 receptor effectors AI229237 Opioid receptor-like Y11433 Pyrimidinergic receptor P2Y4 M64299 Adenosine A1 receptor E00001 Pro-insulin M29014 Insulin receptor precursor U35315 Serotonin receptor 2C S62043 Serotonin receptor 6 AF000368 Scn9a sodium channel, type IX, alpha polypeptide M27158 Kcna5 K+ voltage-gated channel, shaker-related subfamily, member 5 X17621 Kcna6 potassium voltage-gated channel, shaker-related, subfamily, member 6 X16476 Kcnb1 potassium voltage gated channel, Shab-related subfamily, member 1 M77482 Kcnb2 potassium voltage gated channel, Shab-related subfamily, member 2 S64320 Kcnd2 potassium voltage gated channel, Shal-related family, member 2 Ion channels X87635 Kcnj4 potassium inwardly-rectifying channel, subfamily J, member 4 D86039 Kcnj11 potassium inwardly-rectifying channel, subfamily J, member 11 X83581 Kcnj16 potassium inwardly-rectifying channel, subfamily J, member 16 AF073891 Kcnh5 potassium voltage-gated channel, subfamily H (eag-related), member 5 U69882 Kcnn2 potassium intermediate/small conductance calcium-activated channel, subfamily N, member 2 Z36944 Chloride channel 4-2 Z56277 Chloride channel 5 L08493 GABA-A receptor alpha-4 subunit X51992 -

Characterization of Innexin Gene Expression and Functional Roles of Gap-Junctional Communication in Planarian Regeneration
Developmental Biology 287 (2005) 314 – 335 www.elsevier.com/locate/ydbio Characterization of innexin gene expression and functional roles of gap-junctional communication in planarian regeneration Taisaku Nogi, Michael Levin * Department of Cytokine Biology, The Forsyth Institute, 140 The Fenway, Boston, MA 02115, USA Department of Oral and Developmental Biology, Harvard School of Dental Medicine, 140 The Fenway, Boston, MA 02115, USA Received for publication 17 January 2005, revised 20 August 2005, accepted 1 September 2005 Available online 21 October 2005 Abstract Planaria possess remarkable powers of regeneration. After bisection, one blastema regenerates a head, while the other forms a tail. The ability of previously-adjacent cells to adopt radically different fates could be due to long-range signaling allowing determination of position relative to, and the identity of, remaining tissue. However, this process is not understood at the molecular level. Following the hypothesis that gap-junctional communication (GJC) may underlie this signaling, we cloned and characterized the expression of the Innexin gene family during planarian regeneration. Planarian innexins fall into 3 groups according to both sequence and expression. The concordance between expression-based and phylogenetic grouping suggests diversification of 3 ancestral innexin genes into the large family of planarian innexins. Innexin expression was detected throughout the animal, as well as specifically in regeneration blastemas, consistent with a role in long-range signaling relevant to specification of blastema positional identity. Exposure to a GJC-blocking reagent which does not distinguish among gap junctions composed of different Innexin proteins (is not subject to compensation or redundancy) often resulted in bipolar (2-headed) animals. -

Ion Channels 3 1
r r r Cell Signalling Biology Michael J. Berridge Module 3 Ion Channels 3 1 Module 3 Ion Channels Synopsis Ion channels have two main signalling functions: either they can generate second messengers or they can function as effectors by responding to such messengers. Their role in signal generation is mainly centred on the Ca2 + signalling pathway, which has a large number of Ca2+ entry channels and internal Ca2+ release channels, both of which contribute to the generation of Ca2 + signals. Ion channels are also important effectors in that they mediate the action of different intracellular signalling pathways. There are a large number of K+ channels and many of these function in different + aspects of cell signalling. The voltage-dependent K (KV) channels regulate membrane potential and + excitability. The inward rectifier K (Kir) channel family has a number of important groups of channels + + such as the G protein-gated inward rectifier K (GIRK) channels and the ATP-sensitive K (KATP) + + channels. The two-pore domain K (K2P) channels are responsible for the large background K current. Some of the actions of Ca2 + are carried out by Ca2+-sensitive K+ channels and Ca2+-sensitive Cl − channels. The latter are members of a large group of chloride channels and transporters with multiple functions. There is a large family of ATP-binding cassette (ABC) transporters some of which have a signalling role in that they extrude signalling components from the cell. One of the ABC transporters is the cystic − − fibrosis transmembrane conductance regulator (CFTR) that conducts anions (Cl and HCO3 )and contributes to the osmotic gradient for the parallel flow of water in various transporting epithelia. -

Stem Cells and Ion Channels
Stem Cells International Stem Cells and Ion Channels Guest Editors: Stefan Liebau, Alexander Kleger, Michael Levin, and Shan Ping Yu Stem Cells and Ion Channels Stem Cells International Stem Cells and Ion Channels Guest Editors: Stefan Liebau, Alexander Kleger, Michael Levin, and Shan Ping Yu Copyright © 2013 Hindawi Publishing Corporation. All rights reserved. This is a special issue published in “Stem Cells International.” All articles are open access articles distributed under the Creative Com- mons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Editorial Board Nadire N. Ali, UK Joseph Itskovitz-Eldor, Israel Pranela Rameshwar, USA Anthony Atala, USA Pavla Jendelova, Czech Republic Hannele T. Ruohola-Baker, USA Nissim Benvenisty, Israel Arne Jensen, Germany D. S. Sakaguchi, USA Kenneth Boheler, USA Sue Kimber, UK Paul R. Sanberg, USA Dominique Bonnet, UK Mark D. Kirk, USA Paul T. Sharpe, UK B. Bunnell, USA Gary E. Lyons, USA Ashok Shetty, USA Kevin D. Bunting, USA Athanasios Mantalaris, UK Igor Slukvin, USA Richard K. Burt, USA Pilar Martin-Duque, Spain Ann Steele, USA Gerald A. Colvin, USA EvaMezey,USA Alexander Storch, Germany Stephen Dalton, USA Karim Nayernia, UK Marc Turner, UK Leonard M. Eisenberg, USA K. Sue O’Shea, USA Su-Chun Zhang, USA Marina Emborg, USA J. Parent, USA Weian Zhao, USA Josef Fulka, Czech Republic Bruno Peault, USA Joel C. Glover, Norway Stefan Przyborski, UK Contents Stem Cells and Ion Channels, Stefan Liebau, -

An Update on Connexin Gap Junction and Hemichannels in Diabetic Retinopathy
International Journal of Molecular Sciences Review An Update on Connexin Gap Junction and Hemichannels in Diabetic Retinopathy Jorge González-Casanova 1 , Oliver Schmachtenberg 2, Agustín D. Martínez 3, Helmuth A. Sanchez 3, Paloma A. Harcha 3 and Diana Rojas-Gomez 4,* 1 Instituto de Ciencias Biomédicas, Facultad de Ciencias de la Salud, Universidad Autónoma de Chile, Santiago 8910060, Chile; [email protected] 2 Centro Interdisciplinario de Neurociencia de Valparaíso, Instituto de Biología, Facultad de Ciencias, Universidad de Valparaíso, Valparaíso 2360102, Chile; [email protected] 3 Centro Interdisciplinario de Neurociencia de Valparaíso, Instituto de Neurociencia, Facultad de Ciencias, Universidad de Valparaíso, Valparaíso 2360102, Chile; [email protected] (A.D.M.); [email protected] (H.A.S.); [email protected] (P.A.H.) 4 Escuela de Nutrición y Dietética, Facultad de Medicina, Universidad Andres Bello, Santiago 8370146, Chile * Correspondence: [email protected]; Tel.: +56-2-26618559 Abstract: Diabetic retinopathy (DR) is one of the main causes of vision loss in the working age popu- lation. It is characterized by a progressive deterioration of the retinal microvasculature, caused by long-term metabolic alterations inherent to diabetes, leading to a progressive loss of retinal integrity and function. The mammalian retina presents an orderly layered structure that executes initial but complex visual processing and analysis. Gap junction channels (GJC) forming electrical synapses are present in each retinal layer and contribute to the communication between different cell types. Citation: González-Casanova, J.; In addition, connexin hemichannels (HCs) have emerged as relevant players that influence diverse Schmachtenberg, O.; Martínez, A.D.; physiological and pathological processes in the retina. -

Ion Channels
UC Davis UC Davis Previously Published Works Title THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels. Permalink https://escholarship.org/uc/item/1442g5hg Journal British journal of pharmacology, 176 Suppl 1(S1) ISSN 0007-1188 Authors Alexander, Stephen PH Mathie, Alistair Peters, John A et al. Publication Date 2019-12-01 DOI 10.1111/bph.14749 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology (2019) 176, S142–S228 THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels Stephen PH Alexander1 , Alistair Mathie2 ,JohnAPeters3 , Emma L Veale2 , Jörg Striessnig4 , Eamonn Kelly5, Jane F Armstrong6 , Elena Faccenda6 ,SimonDHarding6 ,AdamJPawson6 , Joanna L Sharman6 , Christopher Southan6 , Jamie A Davies6 and CGTP Collaborators 1School of Life Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK 2Medway School of Pharmacy, The Universities of Greenwich and Kent at Medway, Anson Building, Central Avenue, Chatham Maritime, Chatham, Kent, ME4 4TB, UK 3Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK 4Pharmacology and Toxicology, Institute of Pharmacy, University of Innsbruck, A-6020 Innsbruck, Austria 5School of Physiology, Pharmacology and Neuroscience, University of Bristol, Bristol, BS8 1TD, UK 6Centre for Discovery Brain Science, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2019/20 is the fourth in this series of biennial publications. The Concise Guide provides concise overviews of the key properties of nearly 1800 human drug targets with an emphasis on selective pharmacology (where available), plus links to the open access knowledgebase source of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. -

Innexin Specificity in Neural Development
Research Article 3379 Gap junction proteins are not interchangeable in development of neural function in the Drosophila visual system Kathryn D. Curtin*, Zhan Zhang and Robert J. Wyman Molecular, Cellular and Developmental Biology, Yale University, New Haven, CT 06511, USA *Author for correspondence (e-mail: [email protected]) Accepted 12 June 2002 Journal of Cell Science 115, 3379-3388 (2002) © The Company of Biologists Ltd Summary Gap junctions (GJs) are composed of proteins from two Specifically, we tested several innexins for their ability to distinct families. In vertebrates, GJs are composed of rescue shakB2 and ogre mutant ERGs and found that, by connexins; a connexin hexamer on one cell lines up with and large, innexins are not interchangeable. We mapped a hexamer on an apposing cell to form the intercellular the protein regions required for this specificity by making channel. In invertebrates, GJs are composed of an molecular chimeras between shakB(N) and ogre and unrelated protein family, the innexins. Different testing their ability to rescue both mutants. Each chimera connexins have distinct properties that make them largely rescued either shakB or ogre but never both. Sequences in non-interchangeable in the animal. Innexins are also a the first half of each protein are necessary for functional large family with high sequence homology, and some specificity. Potentially crucial residues include a small functional differences have been reported. The biological number in the intracellular loop as well as a short stretch implication of innexin differences, such as their ability to just N-terminal to the second transmembrane domain. substitute for one another in the animal, has not been Temporary GJs, possibly between the retina and lamina, explored. -

UC Berkeley UC Berkeley Electronic Theses and Dissertations
UC Berkeley UC Berkeley Electronic Theses and Dissertations Title The Effects of Neurosteroids, such as Pregnenolone Sulfate and its receptor, TrpM3 in the Retina. Permalink https://escholarship.org/uc/item/04d8607f Author Webster, Corey Michael Publication Date 2019 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California The Effects of Neurosteroids, such as Pregnenolone Sulfate, and its receptor, TrpM3 in the Retina. By Corey Webster A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Molecular and Cell Biology in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Marla Feller, Chair Professor Diana Bautista Professor Daniella Kaufer Professor Stephan Lammel Fall 2019 The Effects of Neurosteroids, such as Pregnenolone Sulfate, and its receptor, TrpM3 in the Retina. Copyright 2019 by Corey Webster Abstract The Effects of Neurosteroids, such as Pregnenolone Sulfate, and its receptor, TrpM3 in the Retina. by Corey M. Webster Doctor of Philosophy in Molecular and Cell Biology University of California, Berkeley Professor Marla Feller, Chair Pregnenolone sulfate (PregS) is the precursor to all steroid hormones and is produced in neurons in an activity dependent manner. Studies have shown that PregS production is upregulated during certain critical periods of development, such as in the first year of life in humans, during adolescence, and during pregnancy. Conversely, PregS is decreased during aging, as well as in several neurodevelopmental and neurodegenerative conditions. There are several known targets of PregS, such as a positive allosteric modulator NMDA receptors, sigma1 receptor, and as a negative allosteric modulator of GABA-A receptors.