Proceedings of the Indiana Academy of Science
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

United States Patent (19) 11, 3,989,755 Mccoy Et Al
United States Patent (19) 11, 3,989,755 McCoy et al. (45) Nov. 2, 1976 54 PRODUCTION OF OXIMES BY THE (56) References Cited REACTION OF CARBON MONOXDE WITH UNITED STATES PATENTS NTROCOMPOUNDS 2,945,065 7/1960 Donaruma...................... 260/566 A 75) Inventors: John J. McCoy, Media; John G. 3,480,672 11/1969 Kober et al..................... 260/566 A Zajacek, Strafford, both of Pa.; Karl 3,734,964 5/1973 Knifton........................... 260/566. A E. Fuger, Therwil, Switzerland (73) Assignee: Atlantic Richfield Company, Los Primary Examiner-Gerald A. Schwartz Angeles, Calif. Attorney, Agent, or Firm-Delbert E. McCaslin 22 Filed: Feb. 20, 1975 (21) Appl. No.: 551,487 57) ABSTRACT Related U.S. Application Data Production of oximes (and ketones) by contacting at 63) Continuation-in-part of Ser. No. 372,457, June 21, elevated temperatures and pressures, a primary or sec 1973, abandoned. ondary saturated aliphatic nitrocompound with carbon monoxide in the presence of a catalyst comprising me 52 U.S. C. ........................ 260/566 A; 260/586 C; tallic selenium or inorganic selenium compounds and 260/586 R; 260/593 R a base. (51 int. Cl”........................................ C07C 131/04 58) Field of Search........ 260/566 A, 586 A, 586 R, 20 Claims, No Drawings 260/593 R 3,989,755 2 cycloaliphatic nitrocompound is contacted with carbon PRODUCTION OF OXIMES BY THE REACTION OF monoxide at temperatures in the range of from 50° to 200 C. under pressures in the range of from 10 atmo CARBON MONOXIDE WITH NITROCOMPOUNDS spheres to 200 atmospheres in the presence of a sele nium catalyst and a base. -

Developing a Method for Determining the Mass Balance of Selenium and Tellurium Bioprocessed by a Selenium-Resistant Bacterium Grown in The
Developing a Method for Determining the Mass Balance of Selenium and Tellurium Bioprocessed by a Selenium-Resistant Bacterium Grown in the Presence of Selenite or Tellurite __________________ A Thesis Presented to The Faculty of the Department of Chemistry Sam Houston State University ____________________ In Partial Fulfillment Of the Requirements for the Degree of Master of Science ____________________ by Janet Horton Bius December, 2001 Developing a Method for Determining the Mass Balance of Selenium and Tellurium Bioprocessed by a Selenium-Resistant Bacterium Grown in the Presence of Selenite or Tellurite by Janet Horton Bius ____________________ Approved: _____________________________ Thomas G. Chasteen ____________________________ Mary F. Plishker ____________________________ Rick C. White Approved: ____________________________ Brian Chapman, Dean College of Arts and Sciences II ABSTRACT Bius, Janet Horton, Developing a Method for Determining the Mass Balance of Selenium and Tellurium Bioprocessed by a Selenium-Resistant Bacterium Grown in the Presence of Selenite or Tellurite, Master of Science (Chemistry), December, 2001. Sam Houston State University, Hunts- ville, Texas, 68 pp. Purpose The purpose of this investigation was to determine: (1) the mass balance of selenium or tellurium that was bioreduced when a selenium-resistant facultative anaerobe was amended with either selenium or tellurium; and (2) methods to analyze for these metalloids in biological samples. Methods Analytical methods were developed for the determination of -

Is Selenium a Metal, Non-Metal Or Metalloid?
Is Selenium a metal, non-metal or metalloid? Abstract Is selenium(Se) a metal, non-metal, or a metalloid? There are various public opinions circulating around it. Since a long time from now, there are a lot of voices discussing this. Even until now, there is still no consensus about it. So, in this project, we are trying to find out whether selenium is a non-metal, metal or metalloids base on its physical and chemical properties which could be studied in the secondary school combining with the other information from the internet. Principles and hypothesis Studied from the secondary school chemistry, the general properties of metals include being good conductors of heat and electricity, having high melting and boiling points. Non-metals generally have a lower melting point and boiling point than metals and they being poor conductors of heat and electricity, etc. And the physical properties of metalloids are having extremely high melting/ boiling point, and having fair electrical conductivity. On the other hand, the oxides of metal are generally basic whereas the oxide of non-metals and metalloids are generally acidic. We will define selenium’s chemical category based on the above properties. Besides, we would like to introduce the concept of displacement reaction in studying the chemical properties of the chalcogens(e.g. sulphur(S) and selenium(Se)), especially selenium such rarely mentioned element. We can assume selenium is a metal if selenium could displace a metal oxide or a metal could displace selenium (IV) oxide. If selenium is a non-metal, its oxide could be displaced by sulphur which is supposed to be more reactive than selenium in the chalcogen group. -

Selenium Dioxide Sld
SELENIUM DIOXIDE SLD CAUTIONARY RESPONSE INFORMATION 4. FIRE HAZARDS 7. SHIPPING INFORMATION 4.1 Flash Point: 7.1 Grades of Purity: Commercial, 99.5+% Common Synonyms Solid White Sour odor Not flammable 7.2 Storage Temperature: Cool ambient Selenious anhydride 4.2 Flammable Limits in Air: Not flammable Selenium oxide 7.3 Inert Atmosphere: No requirement 4.3 Fire Extinguishing Agents: Currently not Sinks and mixes with water. 7.4 Venting: Open available 7.5 IMO Pollution Category: Currently not available KEEP PEOPLE AWAY. AVOID CONTACT WITH SOLID AND DUST. 4.4 Fire Extinguishing Agents Not to Be Wear goggles, dust respirator, and rubber overclothing (including gloves). Used: Currently not available 7.6 Ship Type: Currently not available Notify local health and pollution control agencies. 4.5 Special Hazards of Combustion 7.7 Barge Hull Type: Currently not available Protect water intakes. Products: Sublimes and forms toxic vapors when heated in fire. 8. HAZARD CLASSIFICATIONS Not flammable. 4.6 Behavior in Fire: Currently not available Fire POISONOUS GASES MAY BE PRODUCED WHEN HEATED. 4.7 Auto Ignition Temperature: Not pertinent 8.1 49 CFR Category: Poison 4.8 Electrical Hazards: Not pertinent 8.2 49 CFR Class: 6.1 CALL FOR MEDICAL AID. 4.9 Burning Rate: Not pertinent 8.3 49 CFR Package Group: I Exposure DUST 8.4 Marine Pollutant: No POISONOUS IF INHALED OR IF SKIN IS EXPOSED. 4.10 Adiabatic Flame Temperature: Currently If inhaled will cause coughing or difficult breathing. not available 8.5 NFPA Hazard Classification: Not listed If in eyes, hold eyelids open and flush with plenty of water. -

Why Nature Chose Selenium Hans J
Reviews pubs.acs.org/acschemicalbiology Why Nature Chose Selenium Hans J. Reich*, ‡ and Robert J. Hondal*,† † University of Vermont, Department of Biochemistry, 89 Beaumont Ave, Given Laboratory, Room B413, Burlington, Vermont 05405, United States ‡ University of WisconsinMadison, Department of Chemistry, 1101 University Avenue, Madison, Wisconsin 53706, United States ABSTRACT: The authors were asked by the Editors of ACS Chemical Biology to write an article titled “Why Nature Chose Selenium” for the occasion of the upcoming bicentennial of the discovery of selenium by the Swedish chemist Jöns Jacob Berzelius in 1817 and styled after the famous work of Frank Westheimer on the biological chemistry of phosphate [Westheimer, F. H. (1987) Why Nature Chose Phosphates, Science 235, 1173−1178]. This work gives a history of the important discoveries of the biological processes that selenium participates in, and a point-by-point comparison of the chemistry of selenium with the atom it replaces in biology, sulfur. This analysis shows that redox chemistry is the largest chemical difference between the two chalcogens. This difference is very large for both one-electron and two-electron redox reactions. Much of this difference is due to the inability of selenium to form π bonds of all types. The outer valence electrons of selenium are also more loosely held than those of sulfur. As a result, selenium is a better nucleophile and will react with reactive oxygen species faster than sulfur, but the resulting lack of π-bond character in the Se−O bond means that the Se-oxide can be much more readily reduced in comparison to S-oxides. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Oxidation Mechanism of Copper Selenide
DOI 10.1515/htmp-2013-0097 High Temp. Mater. Proc. 2014; 33(5): 469 – 476 Pekka Taskinen*, Sonja Patana, Petri Kobylin and Petri Latostenmaa Oxidation Mechanism of Copper Selenide Abstract: The oxidation mechanism of copper selenide atmosphere [2]. The roasting option is industrially attrac- was investigated at deselenization temperatures of copper tive as it leads to essentially 100% separation of selenium refining anode slimes. The isothermal roasting of syn- from the other components of anode slime and produces thetic, massive copper selenide in flowing oxygen and relatively pure crude selenium in a single step. oxygen – 20% sulfur dioxide mixtures at 450–550 °C indi- The industrial copper anode slimes are complex mix- cate that in both atmospheres the mass of Cu2Se increases tures of the insoluble substances in the electrolyte and a as a function of time, due to formation of copper selenite significant fraction of it comes from residues of the mold as an intermediate product. Copper selenide oxidises to paint in the anode casting, typically barium sulfate. Sele- copper oxides without formation of thick copper selenite nium is present in the slimes fed to the deselenization scales, and a significant fraction of selenium is vaporized process mostly as copper and silver selenides as well as as SeO2(g). The oxidation product scales on Cu2Se are elementary selenium [3], depending on the processing porous which allows transport of atmospheric oxygen to steps prior to the actual selenium roasting. Therefore, the reaction zone and selenium dioxide vapor to the their detailed mineralogical analysis is not straightfor- surrounding gas. Predominance area diagrams of the ward on the microscopic scale. -

C2cc33754a.Pdf
Electronic Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2012 Supporting Information for …Synthesis of Selenium Nano-Composite (t-Se@PS) by Surface Initiated Atom Transfer Radical Polymerization … by Michael C. P. Wang, and Byron D. Gates* Supporting Information for … Synthesis of Selenium Nano-Composite (t-Se@PS) by Surface Initiated Atom Transfer Radical Polymerization Michael C. P. Wang, and Byron D. Gates* Department of Chemistry and 4D LABS, Simon Fraser University, 8888 University Drive, Burnaby, BC, V5A 1S6, Canada * To whom correspondence should be addressed. Email: [email protected] Tel. (+1) 778-782-8066. Fax. (+1) 778-782-3765. 1 Electronic Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2012 Supporting Information for …Synthesis of Selenium Nano-Composite (t-Se@PS) by Surface Initiated Atom Transfer Radical Polymerization … by Michael C. P. Wang, and Byron D. Gates* Experimental Anisotropic nanostructures of single crystalline t-Se were synthesized by a hydrothermal process without the assistance of surfactants, which are commonly used to facilitate the formation of anisotropic nanostructures.1-2 Surfactants are used in other syntheses to prevent the nanostructures from aggregating, and serve as protective barriers between nanostructures’ surfaces and the surrounding chemical environment. The absence of surfactants or other coatings on the surfaces of the selenium nanostructures expose its surfaces to oxidative damage by oxygen and moisture. Synthesis of 1D t-Se nanostructures:3 One dimensional selenium nanostructures were synthesized from amorphous selenium colloids. The selenium colloids were synthesized by reducing selenious acid with hydrazine. For example, 2.73 g (21.1 mmol) of selenious acid (H2SeO3; 98%; Sigma-Aldrich) was dissolved in 100 mL of 18 Mcm water (purified with Barnstead Nanopure DIamond Life Science water filtration system) with magnetic stirring in a 250 mL round bottom flask. -

Unique Properties of Aluminium and Its Compounds
Unique properties of some selected p block elements (Reading material for CML 514) Unique properties of Aluminium and its compounds Aluminium is the third most abundant metal on the earth’s crust and it is found to be present in as many as 270 minerals. Aluminosilicates form a major component of the earth’s crust. Aluminium is one of the lightest metals with low density and resists reactivity by forming a thin passivation layer of aluminium oxide (about 4 nm thickness) on any exposed aluminium surface. Although pure aluminium has a very low yield strength (the stress at which a material begins to deform plastically) its alloys with metals such as copper, magnesium and zinc have very high yield strength comparable to that of steel. Many of its alloys have very good strength to weight ratio and therefore find applications where a strong light metal is required such as aeroplanes, modern trains and racing cars. It is a good thermal and electrical conductor, possessing 59% of the electrical conductivity of copper, while its density is only 30% that of copper. AlCl3 is a very good Lewis acid and a reagent suited specifically for alkylation and acylation reactions of aromatics (Friedel–Crafts reaction). Sodium salts of long chain aryl sulphonates (for example, dodecyl benzenesulphonate), whose aryl–alkyl unit is made using AlCl3 catalyst, are used as surfactants in laundry detergents. Aluminium sulfate is an important flocculating agent in the purification of water and a sizing agent of paper replacing potassium alum. Positively charged aluminium cations neutralise the negative charge on colloidal particles in water leading to mutual adsorption and flocculation. -

Selenium Recovery in Precious Metal Technology
Selenium Recovery in Precious Metal Technology Fabian Kling Willig Degree Project in Engineering Chemistry, 30 hp Report passed: February 2014 Supervisors: Tomas Hedlund, Umeå University Mikhail Maliarik, Outotec (Sweden) AB Abstract Selenium is mostly extracted from copper anode slimes because the selenium-rich ores are too rare to be mined with profit. When copper anode slimes are processed in a precious metal plant the first step is to remove copper by pressure leach in an autoclave. The anode slime is then dried and fed into a Kaldo furnace. The Kaldo is heated and reduction and smelting begins. During reduction, slag builders are added to form a slag with impurities and fluxes with coke breeze are added to reduce precious metal. In the oxidation step, selenium dioxide, sulphur dioxide and some tellurium dioxide are removed along with the process gas. The dioxides and the process gas are captured in a circulating venturi solution in the gas cleaning system. The dioxides lowers the pH of the venturi solution. Sodium hydroxide is added to the solution to keep pH above 4 in order to prevent selenium from precipitating in the circulation tank. After a Kaldo cycle, the venturi solution is transferred to a precipitation tank where sulphur dioxide is added in order to precipitate selenium. The precipitated selenium is finally collected in a filter press and sold as crude (99.5%) selenium. During commissioning of a precious metal plant and during processing of 2 batches anode slime, data such as pH and electrochemical potential was collected from the venturi solution and later shown in a Pourbaix diagram. -
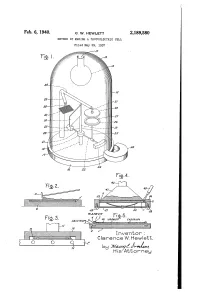
Is Attorney Patented Feb
Feb. 6, 1940. ` Q_ W, HEWLETT 2,189,580 METHOD OF MAKING A PHOTOELECTRIC CELL Filed May 29, 1937 Inventor : Clarence VV. Hewlett, is Attorney Patented Feb. 6, 1940 , 2,189,580 UNITED’ STATES PATENT OFFICE _, g aisance METHOD 0F MAKING A PHUTOELECTRIC Clarence W. Hewlett, Schenectady, N. Y., assigner to General Electric Company, a corporation of New York Application May 29, 1937, Serial No. 145,539 4 Claims. (CLES-8.9) The present invention relates to light-sensitive posited .on the selenium. The first, which is devices, more particularly to photoelectric cells, designated by reference character 4, is preferably which generate a measurable electromotive force of cadmium, and the uppermost metal layer 6 is when subjected to light of practical intensities. a relatively non-oxidizable metal preferably 5 In my application Serial No. 716,677, filed platinum. A contact ring 6 of any suitable metal March 21, 1934, I have disclosed and claimed a such as cadmium is applied in any convenient photo-voltaic cell which consists of a metal plate manner either to the selenium layer 3 before the or base member coated first with selenium and sputtered layers have been deposited, or to the then with cadmium and a relatively non-oxidiz platinum 5 after both metal layers have been de l0 able metal such as platinum in order. The cad posited. In case the ring is applied to the se 10 mium and platinum layers are preferably laid lenium layer, the sputtered layers are deposited down by‘sputtering and are so t-hin as to be semi not only over the selenium layer but also over ` transparent to light.