Characterization of the Essential Oil from Cone-Berries of Juniperus Communis L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Juniperus Communis L.) Essential Oil
Antioxidants 2014, 3, 81-98; doi:10.3390/antiox3010081 OPEN ACCESS antioxidants ISSN 2076-3921 www.mdpi.com/journal/antioxidants Article Chemical Composition and Antioxidant Properties of Juniper Berry (Juniperus communis L.) Essential Oil. Action of the Essential Oil on the Antioxidant Protection of Saccharomyces cerevisiae Model Organism Martina Höferl 1,*, Ivanka Stoilova 2, Erich Schmidt 1, Jürgen Wanner 3, Leopold Jirovetz 1, Dora Trifonova 2, Lutsian Krastev 4 and Albert Krastanov 2 1 Department of Pharmaceutical Chemistry, Division of Clinical Pharmacy and Diagnostics, University of Vienna, Vienna 1090, Austria; E-Mails: [email protected] (E.S.); [email protected] (L.J.) 2 Department Biotechnology, University of Food Technologies, Plovdiv 4002, Bulgaria; E-Mails: [email protected] (I.S.); [email protected] (D.T.); [email protected] (A.K.) 3 Kurt Kitzing Co., Wallerstein 86757, Germany; E-Mail: [email protected] 4 University Laboratory for Food Analyses, University of Food Technologies, Plovdiv 4002, Bulgaria; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +43-1-4277-55555; Fax: +43-1-4277-855555. Received: 11 December 2013; in revised form: 26 January 2014 / Accepted: 28 January 2014 / Published: 24 February 2014 Abstract: The essential oil of juniper berries (Juniperus communis L., Cupressaceae) is traditionally used for medicinal and flavoring purposes. As elucidated by gas chromatography/flame ionization detector (GC/FID) and gas chromatography/mass spectrometry (GC/MS methods), the juniper berry oil from Bulgaria is largely comprised of monoterpene hydrocarbons such as α-pinene (51.4%), myrcene (8.3%), sabinene (5.8%), limonene (5.1%) and β-pinene (5.0%). -

Influence of Tea Tree Essential Oil and Poly(Ethylene Glycol)
materials Article Influence of Tea Tree Essential Oil and Poly(ethylene glycol) on Antibacterial and Physicochemical Properties of Polylactide-Based Films Iwona Tarach 1, Ewa Olewnik-Kruszkowska 1,* , Agnieszka Richert 2 , Magdalena Gierszewska 1 and Anna Rudawska 3 1 Chair of Physical Chemistry and Physicochemistry of Polymers, Faculty of Chemistry, Nicolaus Copernicus University in Toru´n,Gagarina 7 Street, 87-100 Toru´n,Poland; [email protected] (I.T.); [email protected] (M.G.) 2 Chair of Genetics, Faculty of Biological and Veterinary Sciences, Nicolaus Copernicus University in Toru´n, Lwowska 1 Street, 87-100 Toru´n,Poland; [email protected] 3 Department of Production Engineering, Faculty of Mechanical Engineering, Lublin University of Technology, 20-618 Lublin, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-56-611-2210 Received: 5 October 2020; Accepted: 1 November 2020; Published: 4 November 2020 Abstract: The aim of the study was to establish the influence of poly(ethylene glycol) (PEG) on the properties of potential biodegradable packaging materials with antibacterial properties, based on polylactide (PLA) and tea tree essential oil (TTO). The obtained polymeric films consisted of PLA, a natural biocide, and tea tree essential oil (5–20 wt. %) was prepared with or without an addition of 5 wt. % PEG. The PLA-based materials have been tested, taking into account their morphology, and their thermal, mechanical and antibacterial properties against Staphylococcus aureus and Escherichia coli. It was established that the introduction of a plasticizer into the PLA–TTO systems leads to an increase in tensile strength, resistance to deformation, as well an increased thermal stability, in comparison to films modified using only TTO. -

Melissa Officinalis L., a Valuable Medicine Plant: a Review
Journal of Medicinal Plants Research Vol. 4(25), pp. 2753-2759, 29 December Special Review, 2010 Available online at http://www.academicjournals.org/JMPR ISSN 1996-0875 ©2010 Academic Journals Review Melissa officinalis L., a valuable medicine plant: A review Moradkhani H.1, Sargsyan E.1, Bibak H.2, Naseri B.3, Sadat-Hosseini M.2, Fayazi-Barjin A.4 and Meftahizade H.5* 1Institute of Hydroponic Problems, National Academic of Sciences, Yerevan, Republic of Armenia. 2Department of plant production, faculty of Agriculture, university of Jiroft, Kerman, Iran. 3Faculty of Islamic Azad University, Ilam, Iran. 4Department of Plant Protection, University of Tehran, Iran. 5Researcher of ACECR Medicinal Plants Center, Ilam, Iran. Accepted 6 December, 2010 Melissa officinalis L., a valuable medicinal plant in herbal medicine is native to the eastern Mediterranean Region and western Asia. The constituent of the essential oil of the plant in various climates is different, but citral (geranial and neral), citronellal, geraniol are main components. Many parameters influencing essential oil composition and yield, such as light intensity, nutrient, temperature, cultural practice genotype, plant part age, harvesting time. Lemon balm has been traditionally used for different medical purposes as tonic, antispasmodic, carminative, diaphoretic, surgical dressing for wounds, sedative-hypnotic strengthening the memory, and relief of stress induced headache, but in modern pharmacology is value in the management of mild to moderate Alzheimer’s, against migraine and rheumatism, antitumel and antioxidant activities. Key words: Melissa officinalis, essential oil, pharmacology and antioxidant. INTRODUCTION Lemon balm, member of the family Lamiaceae (formerly years may no longer germinate (Zargari, 1991). Labiatae) is a perennial bushy plant and is upright, Lemon balm has a hairy root system with many lateral reaching a height of about 1 m. -

Essential Oils for Beginners Essential Oils for Beginners
Essential Oils for Beginners Essential Oils for Beginners Introduction The Power of the Entire Earth in One Bottle Our planet is a complicated system, made up of intricate ecosystems, endless molecules, and powerful elements. Over the span of 4.5 billion years, the Earth has formed colossal mountains, green valleys, leafy jungles, and vast forests, providing a home for countless plants and animals. On the 196,900,000 square miles of the planet’s surface, scientists estimate that there are 390,000 plants—with new species being discovered all the time. The hundreds of thousands of plants actually perform a number of important functions for the planet. Plants help control the climate, process carbon dioxide and release oxygen into the air to help us breathe, keep living organisms alive, and can be used for food and health solutions, among many other practical uses. With so many benefits, it is no surprise that plants have been used since the beginning of time to help humans perform everyday tasks. 2 Essential Oils for Beginners Ancient times People of ancient civilizations used entire plants, extracts, and various plant parts to make life easier, transforming the gifts of the earth into everything from textiles to remedies. Ancient people believed that the earth gave them everything they needed to solve all of their problems. Present day Fast-forward to modern day. Plants are still everywhere around us, yet with new inventions and technology, we’ve grown accustomed to using synthetic products rather than the gifts of nature to solve everyday challenges. Technology has made life easier, but along with technological advancements, we’ve seen a decline in many areas of quality of life. -
Basic Principles of Blending
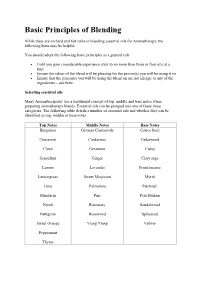
Basic Principles of Blending While there are no hard and fast rules in blending essential oils for Aromatherapy, the following hints may be helpful. You should adopt the following basic principles as a general rule Until you gain considerable experience stick to no more than three or four oils at a time Ensure the odour of the blend will be pleasing for the person(s) you will be using it on Ensure that the person(s) you will be using the blend on are not allergic to any of the ingredients – ask them Selecting essential oils Many Aromatherapists’ use a traditional concept of top, middle and base notes when preparing aromatherapy blends. Essential oils can be grouped into one of these three categories. The following table details a number of essential oils and whether they can be identified as top, middle or base notes. Top Notes Middle Notes Base Notes Bergamot German Chamomile Carrot Seed Cinnamon Cardamom Cedarwood Clove Geranium Cistus Grapefruit Ginger Clary sage Lemon Lavender Frankincense Lemongrass Sweet Marjoram Myrrh Lime Palmarosa Patchouli Mandarin Pine Peru Balsam Neroli Rosemary Sandalwood Petitgrain Rosewood Spikenard Sweet Orange Ylang Ylang Vetiver Peppermint Thyme Top notes are usually fresh, citrus and light. These form the blend’s initial impression, giving brightness and clarity to it. They usually have refreshing and uplifting therapeutic properties. Middle notes (or heart notes) last longer, imparting the warmth and fullness of the blend. They give body to a blend and are mostly found in essential oils distilled from leaves and herbs. Base notes are the heavy smelling, deeply resonating and have a profound influence on the blend. -

Evaluation of Essential Oils and Extracts of Rose Geranium and Rose Petals As Natural Preservatives in Terms of Toxicity, Antimicrobial, and Antiviral Activity
pathogens Article Evaluation of Essential Oils and Extracts of Rose Geranium and Rose Petals as Natural Preservatives in Terms of Toxicity, Antimicrobial, and Antiviral Activity Chrysa Androutsopoulou 1, Spyridoula D. Christopoulou 2, Panagiotis Hahalis 3, Chrysoula Kotsalou 1, Fotini N. Lamari 2 and Apostolos Vantarakis 1,* 1 Department of Public Health, Faculty of Medicine, University of Patras, 26504 Patras, Greece; [email protected] (C.A.); [email protected] (C.K.) 2 Laboratory of Pharmacognosy & Chemistry of Natural Products, Department of Pharmacy, University of Patras, 26504 Patras, Greece; [email protected] (S.D.C.); [email protected] (F.N.L.) 3 Tentoura Castro-G.P. Hahalis Distillery, 26225 Patras, Greece; [email protected] * Correspondence: [email protected] Abstract: Essential oils (EOs) and extracts of rose geranium (Pelargonium graveolens) and petals of rose (Rosa damascena) have been fully characterized in terms of composition, safety, antimicrobial, and antiviral properties. They were analyzed against Escherichia coli, Salmonella enterica serovar Typhimurium, Staphylococcus aureus, Aspergillus niger, and Adenovirus 35. Their toxicity and life span were also determined. EO of P. graveolens (5%) did not retain any antibacterial activity (whereas at Citation: Androutsopoulou, C.; 100% it was greatly effective against E. coli), had antifungal activity against A. niger, and significant Christopoulou, S.D.; Hahalis, P.; antiviral activity. Rose geranium extract (dilutions 25−90%) (v/v) had antifungal and antibacterial Kotsalou, C.; Lamari, F.N.; Vantarakis, activity, especially against E. coli, and dose-dependent antiviral activity. Rose petals EO (5%) retains A. Evaluation of Essential Oils and low inhibitory activity against S. aureus and S. Typhimurium growth (about 20−30%), antifungal Extracts of Rose Geranium and Rose activity, and antiviral activity for medium to low virus concentrations. -

Health Benefits of Geranium Essential Oil
Health Benefits of Geranium Essential Oil The health benefits of Geranium Essential Oil can be attributed to its properties like astringent, haemostatic, cicatrisant, cytophylactic, diuretic, deodorant, styptic, tonic, vermifuge and vulnerary etc. The Essential Oil of Geranium is extracted through steam distillation of stem and leaves of Geranium plant, bearing scientific name Pelargonium Odorantissimum. The main components of this oil are Alpha Pinene, Myrcene, Limonene, Menthone, Linalool, Geranyl Acetate, Citronellol, Geraniol and Geranyl Butyrate. The Essential Oil of Geranium has a lot to offer in terms of health. We can benefit from the following properties of Geranium Oil. • Astringent: The main function of an astringent is to induce contractions. Accordingly Geranium Oil, being an astringent, makes the gums, muscles (including contraction of abdominal muscles which gives you a better look), intestines, skin, tissues and blood vessels to contract. This may be helpful in many ways. It can prevent muscles and skin from hanging loose, untimely loosening and fall of teeth, wrinkles and can even stop haemorrhage by contracting blood vessels. • Anti Bacterial & Anti Microbial: This property does not let bacteria or microbes develop on wounds and otherwise and keeps you safe from infections. • Haemostatic: Geranium Oil can stop haemorrhage in two ways. First, being an astringent (more specifically, a styptic), it causes contraction of blood vessels and helps stop flow of blood, as discussed above. Second, being a Haemostatic, it speeds up coagulation or clotting of blood. • Cicatrisant: Everybody wants that his or her skin to be free from scars and after marks of fat-cracks, surgeries, boils and acne or pox. -

The Effect of the Rose Essential Oil Aroma on University Students’ Learning and Short-Term Memory: a Randomized Controlled Trial
JOURNAL OF CLINICAL MEDICINE OF KAZAKHSTAN (E-ISSN 2313-1519) Original Article DOI: https://doi.org/10.23950/jcmk/9651 The effect of the rose essential oil aroma on university students’ learning and short-term memory: A randomized controlled trial Elif Ok, Vildan Kocatepe, Vesile Ünver Department of Nursing, School of Health Sciences, Acıbadem Mehmet Ali Aydinlar University, Istanbul, Turkey Abstract Received: 2020-11-08 Aim: This randomized controlled experimental study analyzes the Accepted: 2020-01-05 effect of the rose essential oil aroma on university students’ learning and recalling of information in short-term memory. Material and methods: The study sample consisted of 131 students who had never attended hypoglycemia management education (first year), who had recently attended this education (sophomore), and who had attended this education a long time ago (third year). The J Clin Med Kaz 2021; 18(1):32-37 experimental group was administered a pre-test before the education, and rose essential oil aroma was administered for all tests during and Corresponding author: after the education. The control group only received the education and Elif Ok. was administered the tests. E-mail: [email protected]; Results: No statistically significant differences were found ORCID: https://orcid.org/0000-0003-4342-4965 between the pre-test and post-test mean scores of the experimental and control groups in third year students but the differences between the mean scores on the tests administered on the 7th and 30th days were statistically significant. A statistically significant difference was found between the post-test mean scores obtained by the experimental group students who had (second and third year) and had not (first year) attended this education on the 7th day. -

Price List Is Updated Daily
Disclaimer: This price list is updated daily. Eden Botanicals, LLC Please see our website for the most current information. 3820 Cypress Dr. #12 Petaluma, CA 94954 USA Distilled Essential Oils · Expresed Citrus Oils www.edenbotanicals.com Absolutes - CO2 Extracts · Organic Extracts (Extraits) [email protected] Wildcrafted Essential Oils & Extracts · Rare & Precious Oils Organic Essential Oils · Organic CO2 Extracts · Dilutions Toll Free 1-855-EDENOIL Antioxidants · Carrier Oils · Essence Blends Tel 1-707-509-0041 Containers · Accessories Fax 1-707-949-2526 Eden Botanicals Catalog - Page 1 Updated Sep 24, 2021 COMMON NAME ITEM SAMPLE 5 10 15 ML 30 ML 2 4 8 16 1 (Scientific Name) CODE VIAL ML ML (1/2 OZ) (1 OZ) OZ OZ OZ OZ KG NEWLY ADDED HAS ORIFICE REDUCER IS TINY AGARWOOD 57 $12 $169 / $404 $711 $1,265 $2,299 / / / (Aquilaria crassna) Steam Distilled Essential Oil Use: Aromatherapy/Natural Perfumery/Incense. Rich and complex, sweet, warm, deep, precious woody aroma, shades of smoky, amber-y Origin: Vietnam incense and honeyed tobacco, and animalic notes of musk/castoreum - in a word, amazing! AGARWOOD - 5% 58 $3 $14 / $33 $57 $100 $178 $320 $580 $1,167 (Aquilaria crassna) Steam Distilled Essential Oil Use: Aromatherapy/Natural Perfumery/Incense. Rich and complex, sweet, warm, deep, precious woody aroma, shades of smoky, amber-y Origin: Vietnam incense and honeyed tobacco, and animalic notes of musk/castoreum - in a word, amazing! ALMOND, BITTER 59 $3 $20 / $46 $80 $142 $253 $455 / / (Prunus armeniaca L.) Steam Distilled Essential Oil Use: Natural Perfumery. Prussic acid has been removed, making this oil non-toxic for use in perfumery. -

Antimicrobial Activity of Juniper Berry Essential Oil (Juniperus Communis L., Cupressaceae)
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/7395099 Antimicrobial activity of juniper berry essential oil (Juniperus communis L., Cupressaceae) Article in Acta Pharmaceutica · January 2006 Source: PubMed CITATIONS READS 79 589 4 authors, including: Stjepan Pepeljnjak Ivan Kosalec University of Zagreb University of Zagreb 132 PUBLICATIONS 2,173 CITATIONS 118 PUBLICATIONS 1,870 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Chemical Fingerprint of plant-derived materials and biological activities evaluation View project The inhibition of candida's virulence factors in vitro by defined molecules with anti-inflammatory or/and immuno-modulatory effects View project All content following this page was uploaded by Stjepan Pepeljnjak on 05 June 2014. The user has requested enhancement of the downloaded file. Acta Pharm. 55 (2005) 417–422 Short communication Antimicrobial activity of juniper berry essential oil (Juniperus communis L., Cupressaceae) STJEPAN PEPELJNJAK1* Juniper essential oil (Juniperi aetheroleum) was obtained IVAN KOSALEC1 2 from the juniper berry, and the GC/MS analysis showed ZDENKA KALO\ERA a NIKOLA BLA@EVI]3 that the main compounds in the oil were -pinene (29.17%) and b-pinene (17.84%), sabinene (13.55%), limonene (5.52%), 1 Department of Microbiology and mircene (0.33%). Juniper essential oil was evaluated Faculty of Pharmacy and Biochemistry for the antimicrobial activity against sixteen -

INCI Terminology
www.WholesaleSuppliesPlus.com 1(800)359-0944 INCI TERMINOLOGY - SINGLE INGREDIENT COMMON NAME INCI TERM Agar Agar Gelidium Amansii (Agar) Alcohol/Denatured Alcohol/SDA Alcohol Alfalfa Powder Medicago Sativa (Alfalfa) Leaf Powder Alkanet/Alkanet Root Alkanna Tinctoria Root Extract Allantoin Allantoin Almond Meal Prunus Dulcis (Almond) Meal Almond Milk Prunus Dulcis (Almond) Milk Almond Oil/Sweet Almond Oil Prunus Dulcis (Almond) Oil Aloe Extract Butter Cocos Nucifera (Coconut) Oil (and) Aloe Barbadensis Leaf Extract Aloe Vera 100x Aloe Barbadensis Leaf Juice (and) Maltodextrin Aloe Vera 200x Aloe Barbadensis Leaf Juice Aloe Vera Extract Aloe Barbadensis (Aloe) Leaf Extract Aloe Vera Gel Aloe Barbadensis (Aloe) Leaf Juice Aloe Vera Juice Aloe Barbadensis (Aloe) Leaf Juice Alum Amyris balsamifera (Amyris) Oil Amyris Essential Oil Amyris balsamifera (Amyris) Oil Anise Essential Oil Pimpinella Anisum (Anise) Oil Anise Powder Pimpinella Anisum (Anise Annatto Annatto (Bixa Orelana) Annatto Powder Annatto (Bixa orelana) Apricot Kernel Oil Prunus Armeniaca (Apricot) Kernel Oil Apricot Seed Powder Prunus Armeniaca (Apricot) Seed Powder Arnica Arnica Montana (Arnica) Arrowroot Powder Maranta Arundinaceae (Arrowroot) Ascorbic Acid USP/Vitamin C Acorbic Acid Avocado Persea Gratissima (Avocado) Fruit Avocado Oil Persea Gratissima (Avocado) Oil Babassu Oil Orbignya Oleifera (Babassu) Seed Oil Baking Soda Sodium Bicarbonate Balsam Fir Essential Oil Abies Balsamea (Balsam Canda) Resin Balsam Peru/Peru Balsam Essential Oil Myroxylon Pereira (Balsam Peru) -

Tea Tree Oil
CONTACT DERMATITIS Tea Tree Oil Orville Hartford, MD; Kathryn A. Zug, MD Tea tree oil is a popular ingredient in many over- more than 15% 1,8-cineole (eucalyptol).3,4 Although the-counter healthcare and cosmetic products. eucalyptol is not an irritant, eucalyptus essential oil With the explosion of the natural and alternative has eucalyptol as its major component.2 medicine industry, more and more people are using products containing tea tree oil. This arti- Sources and Exposure cle reviews basic information about tea tree oil Mass marketing of Australian tea tree oil as a nat- and contact allergy, including sources of tea tree ural cure for a variety of skin conditions has lead to oil, chemical composition, potential cross reac- its inclusion in products such as cosmetics, sham- tions, reported cases of allergic contact dermati- poos, mouthwashes, ointments, soaps, lotions, tis, allergenic compounds in tea tree oil, deodorants, sunscreens, laundry detergents, tooth- practical patch testing information, and preven- paste, fabric softeners, and cleansers.1 Tea tree oil tive measures. also is used for massage and aromatherapy and can Cutis. 2005;76:178-180. be found in nonprescription medications for the treatment of athlete’s foot, warts, acne, bacterial infections, lice, and psoriasis.5 Finally, tea tree oil is used by the general public and paramedical practi- Allergen Aspects tioners for a myriad of conditions.6 Tea tree oil is an essential oil most often distilled Tea tree oil’s topical antimicrobial activity has from the terminal branches