Quality Attributes of Ginger and Cinnamon Essential Oils from Madagascar Adolfina Koroch*, Lalasoa Ranarivelo, Olivier Behra, H
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tips to Roast Vegetables Spice Guide
Tips to Roast Vegetables • Roast at a high oven temp- 400 to 450 degrees F • Chop vegetables in uniform size so they cook evenly • Don’t over crowd the pan, otherwise they will become soft • Roasting veggies with some oil will help them become crispier • To get the most flavor/crispier roast them on the top rack • Seasoning before putting them in the oven will add flavor • Flip veggies halfway through to ensure even cooking • When roasting multiple types of veggies, ensure they have similar cooking times. Good pairs include: Cauliflower and Broccoli cc Carrots and Broccoli Baby potatoes and Butternut Squash Onions and Bell Peppers Zucchini and Yellow Squash Asparagus and Leeks Spice Guide Table of Contents Spices by Cuisine Herbs and Spices 1 Mexican Coriander, Cumin, oregano, garlic powder, cinnamon, chili powder Herbs and Spices that Pair well with Proteins 2 Caribbean Chicken Fajita Bowl Recipe 3 All spice, nutmeg, garlic powder, cloves, cinnamon, ginger Shelf life of Herbs and Spices 4 French Nutmeg, thyme, garlic powder, rosemary, oregano, Herbs de Provence Spices by Cuisine 5 North African Tips to Roast Vegetables BP Cardamum, cinnamon, cumin, paprika, turmeric, ginger Cajun Cayenne, oregano, paprika, thyme, rosemary, bay leaves, Cajun seasoning Thai Basil, cumin, garlic, ginger, turmeric, cardamum, curry powder Mediterranean Oregano, rosemary, thyme, bay leaves, cardamum, cinnamon, cloves, coriander, basil, ginger Indian Bay leaves, cardamum, cayenne, cinnamon, coriander, cumin, ginger, nutmeg, paprika, turmeric, garam masala, curry powder Middle Eastern Bay leaves, cardamum, cinnamon, cloves, cumin, ginger, coriander, oregano, za’atar, garlic powder 5 Shelf Life of Herbs and Herbs and Spices Spices Herbs Herbs are plants that’s leaves can be used to add flavor to foods. -

Juniperus Communis L.) Essential Oil
Antioxidants 2014, 3, 81-98; doi:10.3390/antiox3010081 OPEN ACCESS antioxidants ISSN 2076-3921 www.mdpi.com/journal/antioxidants Article Chemical Composition and Antioxidant Properties of Juniper Berry (Juniperus communis L.) Essential Oil. Action of the Essential Oil on the Antioxidant Protection of Saccharomyces cerevisiae Model Organism Martina Höferl 1,*, Ivanka Stoilova 2, Erich Schmidt 1, Jürgen Wanner 3, Leopold Jirovetz 1, Dora Trifonova 2, Lutsian Krastev 4 and Albert Krastanov 2 1 Department of Pharmaceutical Chemistry, Division of Clinical Pharmacy and Diagnostics, University of Vienna, Vienna 1090, Austria; E-Mails: [email protected] (E.S.); [email protected] (L.J.) 2 Department Biotechnology, University of Food Technologies, Plovdiv 4002, Bulgaria; E-Mails: [email protected] (I.S.); [email protected] (D.T.); [email protected] (A.K.) 3 Kurt Kitzing Co., Wallerstein 86757, Germany; E-Mail: [email protected] 4 University Laboratory for Food Analyses, University of Food Technologies, Plovdiv 4002, Bulgaria; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +43-1-4277-55555; Fax: +43-1-4277-855555. Received: 11 December 2013; in revised form: 26 January 2014 / Accepted: 28 January 2014 / Published: 24 February 2014 Abstract: The essential oil of juniper berries (Juniperus communis L., Cupressaceae) is traditionally used for medicinal and flavoring purposes. As elucidated by gas chromatography/flame ionization detector (GC/FID) and gas chromatography/mass spectrometry (GC/MS methods), the juniper berry oil from Bulgaria is largely comprised of monoterpene hydrocarbons such as α-pinene (51.4%), myrcene (8.3%), sabinene (5.8%), limonene (5.1%) and β-pinene (5.0%). -

Cilantro Dill Rosemary Ginger Mint Basil
Dill Rosemary Basil Herbs Ginger Cilantro Mint What is an Herb? • Plants that are used as flavoring agents • Leaves, seeds or roots can be used • Usually used in small amounts • Many may be used for medicinal or ornamental purposes Basil Basil • Mint-like annual herb used for cooking, garnish, or medicinal purposes • Readily cross pollinates and several hybrids available • Grown in plots of less than 0.1 acre for local sales • A source of organic insecticide and fungicide • Pests: Japanese beetle; annual weeds • Disease: Botrytis, leaf blight, Sclerotinia blight, Fusarium wilt Mint Mint • Perennial, grown from vegetative material • Multiple harvests from a field, sold fresh • Pests: Loopers and Cutworms • Diseases: Verticillium wilt and Rust • Produced by 15 to 25 commercial growers in Texas • Menthols and esters are distilled from peppermint and spearmint in the Pacific Northwest Cilantro – Soil Preparation • Prefers a light, well-drained, moderately fertile loam or sandy soil • Can tolerate other soil conditions Cilantro - Planting • Will start to bolt when temperatures exceed 85 degrees F • Plant in February for April harvest; September for November harvest • Plant seeds 2 inches apart in rows 12 to 15 inches apart if plan to harvest leaves • Plant seeds 8 inches apart in rows 15 inches apart if plan to harvest seeds Cilantro - Planting • Plant seeds about ¼ to ½ inch deep • About 2,000 seeds per ounce, so don’t purchase a lot of seeds for the season • Weekly planting will ensure continuous crop Cilantro - Fertilizing • Should be fertilized -

Potential Control of Postharvest Gray Mold of Pomegranate Fruits Caused by Botrytis Cinerea
11 Env. Biodiv. Soil Security Vol.1, pp. 145- 156 (2017) Potential Control of Postharvest Gray Mold of Pomegranate Fruits Caused by Botrytis Cinerea Samar A. Allam1, Gabr A. Elkot2, Abdelnaser A. Elzaawely1* and Hassan M. El-Zahaby1 1*Department of Agricultural Botany, Faculty of Agriculture, Tanta University, Tanta, and 2Department of Agricultural Botany, Faculty of Agriculture, Kafr Elsheikh University, Kafr El Sheikh, Egypt . RAY mold rot, caused by Botrytis cinerea, is one of the most economically significant Gpostharvest diseases in pomegranate fruits. The aim of the current study is to evaluate Mangifera indica, Thymus vulgaris, Origanum majorana, Salix mucronata, Cinnamomum cassia and Zingiber officinale extracts and biocontrol agents for controlling gray mold disease on pomegranate. In vitro results showed that T. vulgaris (T.v), C. cassia (C.c) and Z. officinale (Z.o) extracts possessed highly significant antifungal activities as they completely inhibited the radial growth of B. cinerea at the concentrations of 30000, 20000 and 30000 ppm, respectively. The combination of the aforementioned plant extracts and fungicide Flusilazole (Flu) overcomes the potency of Flu or the plant extracts alone specially C.c+Flu at the rate of (2:1; v/v) since it inhibited the radial growth of B. cinerea with 89.6% inhibition compared to the control. The study also proved that using the aforementioned plant extracts alone or in combination with Flu as well as the bacterial antagonists (Bacillus subtilis or Pseudomonas fluorescens) significantly reduced the loss in fruit weight. Furthermore, they also prolonged the storage period of pomegranate fruits and maintained high-quality parameters including soluble solids content and titratable acidity after cold storage at 5±1°C and 90% RH. -

Andrew's Rogan Josh Recipe Ingredients
Andrew’s Rogan Josh Recipe Ingredients: - Garlic/Ginger paste(4 garlic cloves and about 1 inch of fresh ginger chopped if you can’t get the paste - blended with water into a paste) - 2 Tablespoons of oil - 1 lb of meat chopped into cubes (lamb is best but you can use beef or chicken) - 2 or 3 tomatoes (chopped) - 2 onions (finely chopped) - 5 cardamom pods (whole) - 4 cloves - 1 or 2 bay leaves - 5 or 6 black pepper corns - 1 and 1/2 cinnamon sticks - Good teaspoon of cumin seeds - 1 teaspoon of ground coriander - 2 teaspoons of paprika - 1 teaspoon of cayenne pepper - 3 or 4 tablespoons of plain yogurt - 1/4 teaspoon of garam masala (not essential - I usually forget about it) - Juice of 1/2 a lemon Heat the oil and brown the meat cubes. Take the meat out and set it to one side. Put the bay leaves, cloves, cardamom, pepper corns and cinnamon into the oil while it’s still hot. Stir it around until the bay leaves change colour and the cloves begin to swell. Add the onions and fry for 5 minutes or so - until they turn a lovely brown colour. Add the ginger/garlic paste and stir for 30 seconds. Now add the coriander, cumin, cayenne and paprika and stir for another 30 seconds. Return the meat and its juices to the pan and stir for 30 seconds. Add the tomato and after a minute or so add 1 tablespoon of yogurt. After stirring in the yogurt until it’s well blended, add the rest of the yogurt. -

Method Nutrition.Pdf
Cold Press Juice Pure Apple, Spinach, Kale, Cucumber, Celery, Ginger, Lemon Calories (g) Carbs (g) Fat (g) Protein (g) Sodium (mg) Sugar (g) 174 41 1 7 176 17 Vital Apple, Cucumber, Kale, Carrots, Beets, Ginger, Lemon Calories (g) Carbs (g) Fat (g) Protein (g) Sodium (mg) Sugar (g) 229 60 0 4 264 25 Revival Turmeric, Ginger, Filtered Water, Lemon Juice, Agave, Cayenne Extract Calories (g) Carbs (g) Fat (g) Protein (g) Sodium (mg) Sugar (g) 175 46 1 1 4 39 Charolade Lemon Juice, Coconut Water, Filtered Water, Ginger, Maple Syrup, Activated Charcoal, Cayenne Calories (g) Carbs (g) Fat (g) Protein (g) Sodium (mg) Sugar (g) 124 30 0 0 74 28 Drive Yams, Pears, Apples, Carrots, Cinnamon, Maca Calories (g) Carbs (g) Fat (g) Protein (g) Sodium (mg) Sugar (g) 572 140 1 8 161 40 Glow Cantelope, Rose Water Calories (g) Carbs (g) Fat (g) Protein (g) Sodium (mg) Sugar (g) 166 40 1 4 78 38 Seedless Watermelon Juice, Lime Juice, Mint Leaves Calories (g) Carbs (g) Fat (g) Protein (g) Sodium (mg) Sugar (g) 161 41 0 2 0 0 Acai Lemonade Acai, Filtered Water, Lemon Juice, Agave, Chia Seeds Calories (g) Carbs (g) Fat (g) Protein (g) Sodium (mg) Sugar (g) 246 54 5 2 11 45 Pitaya Lemonade Pitaya, Filtered Water, Lemon Juice, Agave, Chia Seeds Calories (g) Carbs (g) Fat (g) Protein (g) Sodium (mg) Sugar (g) 242 57 3 2 3 47 Turmerade Turmeric, Filtered Water, Lemon Juice, Agave, Chia Seeds Calories (g) Carbs (g) Fat (g) Protein (g) Sodium (mg) Sugar (g) 242 56 4 2 2 45 Green Matcha Almond Milk, Spirulina, Matcha Green Tea, Vanilla, Chlorophyll, Cinnamon, Maple -

Influence of Tea Tree Essential Oil and Poly(Ethylene Glycol)
materials Article Influence of Tea Tree Essential Oil and Poly(ethylene glycol) on Antibacterial and Physicochemical Properties of Polylactide-Based Films Iwona Tarach 1, Ewa Olewnik-Kruszkowska 1,* , Agnieszka Richert 2 , Magdalena Gierszewska 1 and Anna Rudawska 3 1 Chair of Physical Chemistry and Physicochemistry of Polymers, Faculty of Chemistry, Nicolaus Copernicus University in Toru´n,Gagarina 7 Street, 87-100 Toru´n,Poland; [email protected] (I.T.); [email protected] (M.G.) 2 Chair of Genetics, Faculty of Biological and Veterinary Sciences, Nicolaus Copernicus University in Toru´n, Lwowska 1 Street, 87-100 Toru´n,Poland; [email protected] 3 Department of Production Engineering, Faculty of Mechanical Engineering, Lublin University of Technology, 20-618 Lublin, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-56-611-2210 Received: 5 October 2020; Accepted: 1 November 2020; Published: 4 November 2020 Abstract: The aim of the study was to establish the influence of poly(ethylene glycol) (PEG) on the properties of potential biodegradable packaging materials with antibacterial properties, based on polylactide (PLA) and tea tree essential oil (TTO). The obtained polymeric films consisted of PLA, a natural biocide, and tea tree essential oil (5–20 wt. %) was prepared with or without an addition of 5 wt. % PEG. The PLA-based materials have been tested, taking into account their morphology, and their thermal, mechanical and antibacterial properties against Staphylococcus aureus and Escherichia coli. It was established that the introduction of a plasticizer into the PLA–TTO systems leads to an increase in tensile strength, resistance to deformation, as well an increased thermal stability, in comparison to films modified using only TTO. -

Melissa Officinalis L., a Valuable Medicine Plant: a Review
Journal of Medicinal Plants Research Vol. 4(25), pp. 2753-2759, 29 December Special Review, 2010 Available online at http://www.academicjournals.org/JMPR ISSN 1996-0875 ©2010 Academic Journals Review Melissa officinalis L., a valuable medicine plant: A review Moradkhani H.1, Sargsyan E.1, Bibak H.2, Naseri B.3, Sadat-Hosseini M.2, Fayazi-Barjin A.4 and Meftahizade H.5* 1Institute of Hydroponic Problems, National Academic of Sciences, Yerevan, Republic of Armenia. 2Department of plant production, faculty of Agriculture, university of Jiroft, Kerman, Iran. 3Faculty of Islamic Azad University, Ilam, Iran. 4Department of Plant Protection, University of Tehran, Iran. 5Researcher of ACECR Medicinal Plants Center, Ilam, Iran. Accepted 6 December, 2010 Melissa officinalis L., a valuable medicinal plant in herbal medicine is native to the eastern Mediterranean Region and western Asia. The constituent of the essential oil of the plant in various climates is different, but citral (geranial and neral), citronellal, geraniol are main components. Many parameters influencing essential oil composition and yield, such as light intensity, nutrient, temperature, cultural practice genotype, plant part age, harvesting time. Lemon balm has been traditionally used for different medical purposes as tonic, antispasmodic, carminative, diaphoretic, surgical dressing for wounds, sedative-hypnotic strengthening the memory, and relief of stress induced headache, but in modern pharmacology is value in the management of mild to moderate Alzheimer’s, against migraine and rheumatism, antitumel and antioxidant activities. Key words: Melissa officinalis, essential oil, pharmacology and antioxidant. INTRODUCTION Lemon balm, member of the family Lamiaceae (formerly years may no longer germinate (Zargari, 1991). Labiatae) is a perennial bushy plant and is upright, Lemon balm has a hairy root system with many lateral reaching a height of about 1 m. -

Essential Oils for Beginners Essential Oils for Beginners
Essential Oils for Beginners Essential Oils for Beginners Introduction The Power of the Entire Earth in One Bottle Our planet is a complicated system, made up of intricate ecosystems, endless molecules, and powerful elements. Over the span of 4.5 billion years, the Earth has formed colossal mountains, green valleys, leafy jungles, and vast forests, providing a home for countless plants and animals. On the 196,900,000 square miles of the planet’s surface, scientists estimate that there are 390,000 plants—with new species being discovered all the time. The hundreds of thousands of plants actually perform a number of important functions for the planet. Plants help control the climate, process carbon dioxide and release oxygen into the air to help us breathe, keep living organisms alive, and can be used for food and health solutions, among many other practical uses. With so many benefits, it is no surprise that plants have been used since the beginning of time to help humans perform everyday tasks. 2 Essential Oils for Beginners Ancient times People of ancient civilizations used entire plants, extracts, and various plant parts to make life easier, transforming the gifts of the earth into everything from textiles to remedies. Ancient people believed that the earth gave them everything they needed to solve all of their problems. Present day Fast-forward to modern day. Plants are still everywhere around us, yet with new inventions and technology, we’ve grown accustomed to using synthetic products rather than the gifts of nature to solve everyday challenges. Technology has made life easier, but along with technological advancements, we’ve seen a decline in many areas of quality of life. -

(Cinnamomum Zeylanicum Blume.) & Naluka/Cassia
International Journal of Research in Pharmacy and Biosciences Volume 2, Issue 2, February 2015, PP 1-4 ISSN 2394-5885 (Print) & ISSN 2394-5893 (Online) Identification of Adulterants by Pharmacognostical Evaluation: Tvak (Cinnamomum Zeylanicum Blume.) & Naluka/Cassia [Cinnamomum Cassia (Nees & T.Nees.) J.Presl] Nanda Amalesh1, Paul Nirankush1, Gupta Amartya Kumar1, Ganguly Partha*1, Banerjee Dipankar1, Singh Rahul1, Katiyar Chandrakant1 1R&D Healthcare Division, Emami Ltd., 13 B.T. Road, Belghoria, Kolkata-700 056, India ABSTRACT Traditional medical systems remain important resources of healthcare worldwide that are reported to be safe and produce minimum side effects compared to synthetic medicines. At present deforestation and extinction of many species and incorrect identification of many plants has resulted in adulteration of raw drugs which results in lesser efficacy of the finished formulations? This article throws light on the concepts of adulteration of Naluka/Cassia (Cinnamomum cassia) with Tvak (Cinnamomum zeylanicum). Morphologically near about same, and Tvak is typically more expensive than the Naluka, so it is easy to adulterate Tvak with inferior variety of Naluka to reduce the cost. But there is no instantaneous ready method available to distinguish between Tvak and Naluka. A rough distinction can be made between Tvak and Naluka depending upon the Coumarin content [1] which requires sophisticated analytical technique which involves both time and cost. In the present study, an easy attempt has been made to distinguish between Tvak and Naluka using simple pharmacognostical evaluation.These simple microscopic and macroscopic characters can be used as an effective tool for the identification of true Tvak sample which will help to maintain the quality of herbal drugs by avoiding adulteration of Naluka. -
Basic Principles of Blending
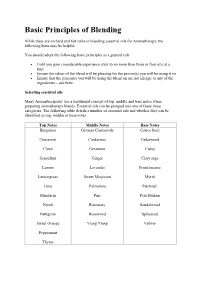
Basic Principles of Blending While there are no hard and fast rules in blending essential oils for Aromatherapy, the following hints may be helpful. You should adopt the following basic principles as a general rule Until you gain considerable experience stick to no more than three or four oils at a time Ensure the odour of the blend will be pleasing for the person(s) you will be using it on Ensure that the person(s) you will be using the blend on are not allergic to any of the ingredients – ask them Selecting essential oils Many Aromatherapists’ use a traditional concept of top, middle and base notes when preparing aromatherapy blends. Essential oils can be grouped into one of these three categories. The following table details a number of essential oils and whether they can be identified as top, middle or base notes. Top Notes Middle Notes Base Notes Bergamot German Chamomile Carrot Seed Cinnamon Cardamom Cedarwood Clove Geranium Cistus Grapefruit Ginger Clary sage Lemon Lavender Frankincense Lemongrass Sweet Marjoram Myrrh Lime Palmarosa Patchouli Mandarin Pine Peru Balsam Neroli Rosemary Sandalwood Petitgrain Rosewood Spikenard Sweet Orange Ylang Ylang Vetiver Peppermint Thyme Top notes are usually fresh, citrus and light. These form the blend’s initial impression, giving brightness and clarity to it. They usually have refreshing and uplifting therapeutic properties. Middle notes (or heart notes) last longer, imparting the warmth and fullness of the blend. They give body to a blend and are mostly found in essential oils distilled from leaves and herbs. Base notes are the heavy smelling, deeply resonating and have a profound influence on the blend. -

Spice Basics
SSpicepice BasicsBasics AAllspicellspice Allspice has a pleasantly warm, fragrant aroma. The name refl ects the pungent taste, which resembles a peppery compound of cloves, cinnamon and nutmeg or mace. Good with eggplant, most fruit, pumpkins and other squashes, sweet potatoes and other root vegetables. Combines well with chili, cloves, coriander, garlic, ginger, mace, mustard, pepper, rosemary and thyme. AAnisenise The aroma and taste of the seeds are sweet, licorice like, warm, and fruity, but Indian anise can have the same fragrant, sweet, licorice notes, with mild peppery undertones. The seeds are more subtly fl avored than fennel or star anise. Good with apples, chestnuts, fi gs, fi sh and seafood, nuts, pumpkin and root vegetables. Combines well with allspice, cardamom, cinnamon, cloves, cumin, fennel, garlic, nutmeg, pepper and star anise. BBasilasil Sweet basil has a complex sweet, spicy aroma with notes of clove and anise. The fl avor is warming, peppery and clove-like with underlying mint and anise tones. Essential to pesto and pistou. Good with corn, cream cheese, eggplant, eggs, lemon, mozzarella, cheese, olives, pasta, peas, pizza, potatoes, rice, tomatoes, white beans and zucchini. Combines well with capers, chives, cilantro, garlic, marjoram, oregano, mint, parsley, rosemary and thyme. BBayay LLeafeaf Bay has a sweet, balsamic aroma with notes of nutmeg and camphor and a cooling astringency. Fresh leaves are slightly bitter, but the bitterness fades if you keep them for a day or two. Fully dried leaves have a potent fl avor and are best when dried only recently. Good with beef, chestnuts, chicken, citrus fruits, fi sh, game, lamb, lentils, rice, tomatoes, white beans.