Non-Commercial Use Only
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Seed Collection Policy and Pricing
NATIVE PLANT SEED COLLECTION PRICING TABLE Note: Prices listed in Vendor prices for Fall/Winter 2012-2013 based on 10% of retail Price Per Pound BLM paid in the recent National seed buys * Common Name TSIS Scientific Name Ecoregion/ Location Fall/Winter ($) * BLM Charge rate ($) SHRUBS Buy 2/Ave Buy 3/Ave Big sagebrush, basin Artemisia tridentata tridentata $ 1.05 $ 2.12 0.159 Big sagebrush, mountain Artemisia tridentata vaseyana $ 1.45 $ 2.95 0.220 Big sagebrush, Wyoming Artemisia tridentata wyomingensis $ 1.06 $ 2.55 0.181 Bitterbrush, antelope Purshia tridentata $ 1.65 $ 1.48 0.157 Bitterbrush, desert Purshia glandulosa $ 1.95 $ 1.70 0.183 Blackbrush Coleogyne ramossisima $ 2.50 0.250 Brittlebush, Virgin River Encelia virginensis $ 0.90 0.090 Bursage, white Ambrosia dumosa $ 2.90 0.290 Cheesebush Hymenoclea salsola $ 2.50 0.250 Cliffrose Cowania stansburiana $ 2.50 0.250 Creosote bush Larrea tridentata $ 3.00 0.300 Ephedra, green Ephedra viridus $ 2.00 0.200 Ephedra, Nevada Ephedra nevadensis $ 2.00 0.200 Mountain mahogany, birchleaf Cercocarpus betulifolia $ 3.00 0.300 Mountain mahogany, curlleaf Cercocarpus ledifolius $ 2.80 $ 2.50 0.265 Mountain mahogany, true Cercocarpus montanus $ 3.00 $ 3.00 0.300 Quailbush Atiplex lentiformis $ 1.25 0.125 Rabbitbrush, rubber Ericameria nauseosa $ 1.15 $ 0.95 0.105 Rabbitbrush, whitestem Ericameria nauseosa ssp. hololeuca $ 1.10 0.110 Sagebrush, black Artemisia nova $ 1.75 $ 1.95 0.185 Sagebrush, low Artemisia arbuscula $ 1.80 $ 1.75 0.178 Saltbush, cattle Atriplex polycarpa $ 1.00 0.100 Saltbush, fourwing Atriplex canescens $ 0.69 $ 0.60 0.065 Shadescale Atriplex confertifolia $ 0.45 $ 0.50 0.048 Winterfat Kraschenninikovia lanata $ 1.20 $ 1.00 0.110 Spiny hopsage Grayia spinosa $ 3.50 0.350 Attachment 4-1 Common Name TSIS Scientific Name Ecoregion/ Location Fall/Winter ($) * BLM Charge rate ($) FORBS Aster/Fleabane Aster or Erigeron sp. -

Simulating the Productivity of Desert Woody Shrubs in Southwestern Texas
DOI: 10.5772/intechopen.73703 ProvisionalChapter chapter 2 Simulating the Productivity of Desert Woody Shrubs in Southwestern Texas SuminSumin Kim, Kim, JaehakJaehak Jeong Jeong and James R. KiniryR. Kiniry Additional information is available at the end of the chapter http://dx.doi.org/10.5772/intechopen.73703 Abstract In the southwestern U.S., many rangelands have converted from native grasslands to woody shrublands dominated by creosotebush (Larrea tridentate) and honey mesquite (Prosopis glandulosa), threatening ecosystem health. Both creosotebush and mesquite have well-developed long root systems that allow them to outcompete neighboring plants. Thus, control of these two invasive shrubs is essential for revegetation in arid rangelands. Simulation models are valuable tools for describing invasive shrub growth and interaction between shrubs and other perennial grasses and for evaluating quantitative changes in ecosystem properties linked to shrub invasion and shrub control. In this study, a hybrid and multiscale modeling approach with two process-based models, ALMANAC and APEX was developed. Through ALMANAC application, plant parameters and growth cycles of creosotebush and mesquite were characterized based on field data. The devel- oped shrub growth curves and parameters were subsequently used in APEX to explore productivity and range condition at a larger field scale. APEX was used to quantitatively evaluate the effect of shrub reductions on vegetation and water and soil qualities in vari- ous topological conditions. The results of this study showed that this multi modeling approach is capable of accurately predicting the impacts of shrubs on soil water resources. Keywords: arid rangeland, creosotebush, mesquite, ALMANAC, APEX 1. Introduction Rangelands cover 31% of the total land base of the U.S. -

CDFG Natural Communities List
Department of Fish and Game Biogeographic Data Branch The Vegetation Classification and Mapping Program List of California Terrestrial Natural Communities Recognized by The California Natural Diversity Database September 2003 Edition Introduction: This document supersedes all other lists of terrestrial natural communities developed by the Natural Diversity Database (CNDDB). It is based on the classification put forth in “A Manual of California Vegetation” (Sawyer and Keeler-Wolf 1995 and upcoming new edition). However, it is structured to be compatible with previous CNDDB lists (e.g., Holland 1986). For those familiar with the Holland numerical coding system you will see a general similarity in the upper levels of the hierarchy. You will also see a greater detail at the lower levels of the hierarchy. The numbering system has been modified to incorporate this richer detail. Decimal points have been added to separate major groupings and two additional digits have been added to encompass the finest hierarchal detail. One of the objectives of the Manual of California Vegetation (MCV) was to apply a uniform hierarchical structure to the State’s vegetation types. Quantifiable classification rules were established to define the major floristic groups, called alliances and associations in the National Vegetation Classification (Grossman et al. 1998). In this document, the alliance level is denoted in the center triplet of the coding system and the associations in the right hand pair of numbers to the left of the final decimal. The numbers of the alliance in the center triplet attempt to denote relationships in floristic similarity. For example, the Chamise-Eastwood Manzanita alliance (37.106.00) is more closely related to the Chamise- Cupleaf Ceanothus alliance (37.105.00) than it is to the Chaparral Whitethorn alliance (37.205.00). -
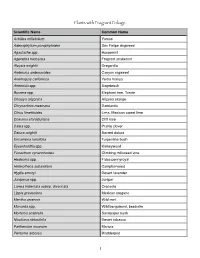
Plants with Fragrant Foliage
Plants with Fragrant Foliage Scientific Name Common Name Achillea millefolium Yarrow Adenophyllum porophyloides San Felipe dogweed Agastache spp. Horsemint Ageratina herbacea Fragrant snakeroot Aloysia wrightii Oreganillo Ambrosia ambrosoides Canyon ragweed Anemopsis californica Yerba mansa Artemisia spp. Sagebrush Bursera spp. Elephant tree, Torote Choisya arizonica Arizona orange Chrysactinia mexicana Damianita Citrus limettioides Lima, Mexican sweet lime Cowania stansburiana Cliff rose Dalea spp. Prairie clover Datura wrightii Sacred datura Ericameria laricifolia Turpentine bush Eysenhardtia spp. Kidneywood Funastrum cynanchoides Climbing milkweed vine Hedeoma spp. False pennyroyal Heterotheca subaxillaris Camphorweed Hyptis emoryi Desert lavender Juniperus spp. Juniper Larrea tridentata subsp. divaricata Creosote Lippia graveolens Mexican oregano Mentha arvensis Wild mint Monarda spp. Wild bergamont, beebalm Mortonia scabrella Sandpaper bush Nicotiana obtusifolia Desert tobacco Parthenium incanum Mariola Peritome arborea Bladderpod !1 Scientific Name Common Name Peucophyllum sschottii Desert fir Poliomentha incana Rosemary mint Poliomentha madrensis Mexican oregano Porophyllum gracile Odora Psorothamnus schottii Schott's smokebush Pseudognaphalium leucocephalum Pearly everlasting Psorothamnus spinosus Smoke tree Ptelea trifoliata Hop tree Rhus aromatica Lemonade berry Salvia spp. Sage Sambucus mexicana Mexican elderberry Stachys coccinea Scarlet betony Stevia lemmonii Santa Catalina candyleaf Tagetes lemmonii Mountain marigold Tagetes lucida Mexican tarragon Tagetes palmeri Sonoran mountain marigold Thelesperma megampotamicum Navajo threadleaf tea Thymophylla acerosa Shrubby dogweed Thymophylla pentacheata Dogweed Trichostemma arizonica Arizona blue curls !2. -

Biogenic Volatile Organic Compound Emissions from Desert Vegetation of the Southwestern US
ARTICLE IN PRESS Atmospheric Environment 40 (2006) 1645–1660 www.elsevier.com/locate/atmosenv Biogenic volatile organic compound emissions from desert vegetation of the southwestern US Chris Gerona,Ã, Alex Guentherb, Jim Greenbergb, Thomas Karlb, Rei Rasmussenc aUnited States Environmental Protection Agency, National Risk Management Research Laboratory, Research Triangle Park, NC 27711, USA bNational Center for Atmospheric Research, Boulder, CO 80303, USA cOregon Graduate Institute, Portland, OR 97291, USA Received 27 July 2005; received in revised form 25 October 2005; accepted 25 October 2005 Abstract Thirteen common plant species in the Mojave and Sonoran Desert regions of the western US were tested for emissions of biogenic non-methane volatile organic compounds (BVOCs). Only two of the species examined emitted isoprene at rates of 10 mgCgÀ1 hÀ1or greater. These species accounted for o10% of the estimated vegetative biomass in these arid regions of low biomass density, indicating that these ecosystems are not likely a strong source of isoprene. However, isoprene emissions from these species continued to increase at much higher leaf temperatures than is observed from species in other ecosystems. Five species, including members of the Ambrosia genus, emitted monoterpenes at rates exceeding 2 mgCgÀ1 hÀ1. Emissions of oxygenated compounds, such as methanol, ethanol, acetone/propanal, and hexanol, from cut branches of several species exceeded 10 mgCgÀ1 hÀ1, warranting further investigation in these ecosystems. Model extrapolation of isoprene emission measurements verifies recently published observations that desert vegetation is a small source of isoprene relative to forests. Annual and daily total model isoprene emission estimates from an eastern US mixed forest landscape were 10–30 times greater than isoprene emissions estimated from the Mojave site. -

Potential of Medicinal Plants As Antimicrobial and Antioxidant Agents in Food Industry: a Hypothesis
Potential of Medicinal Plants as Antimicrobial and Antioxidant Agents in Food Industry: in Food Science A Hypothesis R: Concise Reviews Luis Alberto Ortega-Ramirez, Isela Rodriguez-Garcia, Juan Manuel Leyva, Manuel Reynaldo Cruz-Valenzuela, Brenda Adriana Silva-Espinoza, Gustavo A. Gonzalez-Aguilar, Md Wasim Siddiqui, and Jesus Fernando Ayala-Zavala Abstract: Many food preservation strategies can be used for the control of microbial spoilage and oxidation; however, these quality problems are not yet controlled adequately. Although synthetic antimicrobial and antioxidant agents are approved in many countries, the use of natural safe and effective preservatives is a demand of food consumers and producers. This paper proposes medicinal plants, traditionally used to treat health disorders and prevent diseases, as a source of bioactive compounds having food additive properties. Medicinal plants are rich in terpenes and phenolic compounds that present antimicrobial and antioxidant properties; in addition, the literature revealed that these bioactive compounds extracted from other plants have been effective in food systems. In this context, the present hypothesis paper states that bioactive molecules extracted from medicinal plants can be used as antimicrobial and antioxidant additives in the food industry. Keywords: food safety, natural products, phenolic compounds, terpenes Introduction been used for thousands of years to treat health disorders and The search of new safe substances for food preservation is be- prevent diseases. ing performed -

Principal Plants of Herbal First Aid 7Song, Director Northeast School of Botanical Medicine 7Song.Com
Principal Plants of Herbal First Aid 7Song, Director Northeast School of Botanical Medicine 7Song.com 1. Anemone (Anemone spp.)-For panic, acute anxiety. A useful trauma remedy. Also to help with difficult psychedelic experiences. Internal as tincture. Low dose. 2. Arnica (Arnica spp.)-Antiinflammatory, useful immediately after injury to speed recovery. Internal use as tincture (caution, use drop dose as an internal medicine). External use as oil, liniment. 3. Black cohosh (Actaea racemosa)-For skeletal muscle pain. Avoid in pregnancy. Internal as tincture, capsule. Low to high dose. 4. Chamomile (Matricaria chamomilla)-Antiinflammatory, gut antispasmodic, nervine, anxiolytic, calming agent. Safe, well tolerated. Internal as tea, tincture, glycerite. External as compress. Medium to large dose. 5. Chaparral (Larrea tridentata)- Antibacterial, very bitter. Discontinue after ± 2 weeks with internal use. Internal as tincture, tea, capsule, glycerite. External as soak, oil, liniment. Large loading dose, medium to large maintenance dosage. 6. Echinacea (Echinacea purpurea)-Broad use due to effects on the immune system. Useful in respiratory viruses, wound healing, food poisoning, and bacterial infections. For internal and external infections. Internal as tincture, tea, capsules. External as poultice, compress. Well tolerated. Large loading dose, medium to large maintenance dose. 7. Hops (Humulus lupulus)-Nervine, sedative, sleep aid. Therapeutic use based on dosage. Can be too sedative for individuals. Bitter flavor. Internal as tincture, tea, glycerite. External as oil, liniment. Low to large dose. 8. Jamaican dogwood (Piscidia piscipula)-Pain remedy for general body aches. Helpful used with other pain and sedative remedies. Not very sedative by itself.. Useful with other sleep and pain aids. -

Current Plant List
2317 Evergreen Rd. Current Plant Louisa, Va 23093 (540)967-1165 (434)882-2648 List www.forrestgreenfarm.com COMMON NAME : LATIN NAME COMMON NAME : LATIN NAME COMMON NAME : LATIN NAME African Blue Basil : Calendula : Calendula officinalis Eggplant, Fairy Tale : Ageratum,Blue Planet : Calibrachoa, NOA Black Purple : Elderberry : Sambucus nigra Agrimony : Agrimonia eupatoria Calibrachoa, NOA Orange : Elecampane : Inula helenium Alyssum : Calibrachoa, Ultimate Pink : Evening Primrose : Oenothera biennis Andrographis : Andrographis botrys Cantaloupe, : Everlasting : Helichrysum arenarium Angelica : Angelica archangelica Caraway : Carum carvi Fennel, Bronze & Green : Foeniculum vulgare Angelonia, Purple : Castor Bean : Fennel, Orion : Foeniculum vulgare Angels Trumpet, evening fragrance : Catmint : Nepeta mussinii Feverfew : Tanacetum Parthenium Anise-Hyssop : Agastache foeniculum Catnip : Nepeta Cataria Four O'clock mix : Artichoke, Globe : Cynara scolymus Cauliflower, white : Foxglove : Digitalis purpurea Arugula : Cayenne : Capsicum annuum French Marigold, durango outback mix : Ashwagandha : Withania somnifera Celandine : Chelidonium majus Garden Soil Asian Greens : Celosia, Cramer's Amazon : GarlicHealthy Chives Plants : Allium begin tuberosum with Healthy Soil : Astragalus : Astragalus membranaceus Celosia, Cramer's Burgundy : Gazania, Kiss Orange Flame : Basil, Cinnamon : Chamomile, Common (German) : Matricaria recutita Gentian, Yellow : Gentiana lutea Basil, Dark opal : Chamomile, Roman : Chamaemelum nobile Geranium, Wild : Geranium -

Using Native Plants of the Northeast of Mexico for Developing Active Antimicrobial Food Packaging Films Cecilia Rojas De Gante, Judith A
Chapter Using Native Plants of the Northeast of Mexico for Developing Active Antimicrobial Food Packaging Films Cecilia Rojas de Gante, Judith A. Rocha and Carlos P. Sáenz Collins Abstract The development of active food packaging is addressed using polyolefins such as LDPE and PVOH, as well as biopolymers from flour (sorghum and corn) and by- products of the food industry. Bacteriocins (nisin, natamycin), plant extracts such as oregano and thyme, as well as native plants of the northeast region of Mexico (Larrea tridentata, Schinus molle, Cordia boissieri, Leucophyllum frutescens), and essential oils of oregano and thyme as antimicrobial agents have been studied. The effect exerted by the process of incorporation of the antimicrobial agent (casting, extrusion) on the barrier and mechanical properties of the package as well as the antimicrobial activity of the containers (broad spectrum or selective activity) has been observed and the establishment of methods for their traceability. Keywords: sorghum, maize, flour, nisin, thyme, oregano, Larrea tridentata, Schinus molle, Cordia boissieri, Leucophyllum frutescens, Listeria monocytogenes, Staphylococcus aureus 1. Introduction Since the last decade and a half (2000 to date), the main forces that have unleashed the greatest developments in the packaging of food are the great con- cern of society for the care of their integral health including its nutritional status through foods with less or no presence of additives but in convenient presentations that facilitate their preparation, heating, and intake as well as foods with therapeu- tic action. A consumer who is very concerned about the safety of food, where food packaging and storage systems do not represent or have physical, biological, or even toxicological risks, nor for the protection of the environment. -

Wholesale Catalogue Summer-Fall 2012
Pacific Botanicals Growers & Suppliers of High Quality Certified Organically Grown Herbs & Spices Since 1979 Wholesale Catalogue Summer-Fall 2012 Empowering People Everywhere to Experience the Miracle of Good Health! Mullein in late summer bloom In ThIs Issue: Mark’s Farm Update, Toni’s Window, Vitalizing Herbs for Summer, Skullcap Direct from the Farm, New Guide for Fresh Harvest and Fresh Shipping, The Woman From the Greenhouse, by D.E. Mark’s Farm Update Southwest Oregon is a beautiful part of U.S. We are hoping that the world. I love it here. The green hills, the we can get our first crop evergreens, the lush forests, the many rivers that this year. It takes experi- flow down from the Cascades and out to the sea. ence, research and careful But the price we pay for all of this green is rain. execution to produce a And right now good crop. we are in the We have to put the rainy season. herb through a series of The Apple- trials to determine the gate Valley is ideal conditions for ro- socked in with bust growth. Determining dense, cool, the best seed treatments, persistent Or- potting mix, soil type egon rain. The good news is that our snow pack in the field, cultivation Mark prepares a field for an Echinacea planting is growing and our reservoirs are filling. The rain technique, irrigation requirements, and harvest I hope that you and your family are staying will give us the green landscape we love and crave procedures are several of the many ways we test healthy. -

Additional Species to Know
BIOL 301 Plant Systematics Additional Species for Graduate Students Plants of Significance to North American Agriculture 1. Sunflower, Helianthus annus (Asteraceae) 2. Canola, Brassica napus et al. (Brassicaceae) 3. Wild Cabbage, Brassica oleracea (Brassicaceae) 4. Soybean, Glycine max (Fabaceae) 5. Cotton, Gossypium hirsutum et al. (Malvaceae) 6. Corn, Zea mays subsp. mays and Teosinte, Zea mays subsp. mexicana (Poaceae) 7. Wheat, Triticum aestivum (Poaceae) 8. Rice, Oryza sativa (Poaceae) 9. Strawberry , Fragaria virginiana x Fragaria chiloensis (Rosaceae) 10. Apple, Malus pumila (Rosaceae) 11. Almond, Prunus amygdalus (Rosaceae) 12. Potato, Solanum tuberosum (Solanaceae) 13. Tomato, Solanum lycopersicum (Solanaceae) 14. Tobacco, Nicotiana tabacum (Solanaceae) 15. Grapes, Vitis vinifera et al. (Vitaceae) Plants of Significance to North American Forestry 1. Coastal Redwood, Sequoia sempervirens (Cupressaceae) 2. Giant Sequoia, Sequoiadendron giganteum (Cuppressaceae) 3. Western Red Cedar, Thuja plicata (Cuppressaceae) 4. Red Cedar, Juniperus virgiana (Cupressaceae) 5. Walnut, Juglans nigra (Junglandaceae) 6. Ponderosa Pine, Pinus ponderosa (Pinaceae) 7. White Pine , Pinus strobus (Pinaceae) 8. Douglas Fir, Pseudo menziesii (Pinaceae) 9. Cherry, Prunus serotina (Rosaceae) 10. Poplars (Hybrid Poplars), Populus spp. (Salicaceae) Plants of Major Significance to Ecology/Evolution Research in North America 1. Wild Yarrow, Achillea millefolium (Asteraceae) [classical work on ecological adaptation] 2. Sunflower, Helianthus annuus et al. (Asteraceae) [hybrid speciation, ecological adaptation] 3. Salsifies, Tragopogon miscellus and T. mirus (Asteraceae) [hybrid speciation] 4. Touch-Me-Not, Impatiens capensis (Balsaminaceae) [ecological adaptation, phenotypic plasticity] 5. Mouse-Ear Cress, Arabidopsis thaliana (Brassicaceae) [evolutionary genetics] 6. Boechera, Boechera stricta et al. (Brassicaceae) [evolutionary genetics, ecological adaptation] 7. Soybean, Glycine max et al. (Fabaceae) [evolutionary genetics, polyploid speciation] 8. -

Hierarchical List of Natural Communities with Holland Types
September 2010 Global and State Rank CaCode CNDDB Code 1. Mesomorphic Tree Vegetation (Forest and Woodland) 1.C. Temperate Forest 1.C.1. Warm Temperate Forest 1.C.1.c. Madrean Forest and Woodland MG009. California Forest and Woodland Mixed North Slope Forest G4 S4 CTT81500CA Mixed North Slope Cismontane Woodland G3 S3.2 CTT71420CA *Aesculus californica (California buckeye groves) Alliance G3 S3 *75.100.00 *Aesculus californica *75.100.03 *Aesculus californica - Umbellularia californica / Diplacus aurantiacus *75.100.02 *Aesculus californica - Umbellularia californica / Holodiscus discolor *75.100.06 *Aesculus californica / Datisca glomerata *75.100.04 *Aesculus californica / Lupinus albifrons *75.100.05 *Aesculus californica / Toxicodendron diversilobum / moss *75.100.01 *Juglans californica (California walnut groves) Alliance G3 S3 *72.100.00 California Walnut Woodland G2 S2.1 CTT71210CA Walnut Forest G1 S1.1 CTT81600CA *Juglans californica - Quercus agrifolia *72.100.08 *Juglans californica / annual herbaceous *72.100.03 *Juglans californica / Artemisia californica / Leymus condensatus *72.100.04 *Juglans californica / Ceanothus spinosus *72.100.05 *Juglans californica / Heteromeles arbutifolia *72.100.06 *Juglans californica / Malosma laurina *72.100.07 *Lyonothamnus floribundus (Catalina ironwood groves) Special Stands G2 S2 *77.000.00 Island Ironwood Forest G2 S2.1 CTT81700CA Quercus agrifolia (Coast live oak woodland) Alliance G5 S4 (some associations are of 71.060.00 high priority for inventory) Coast Live Oak Woodland G4 S4 CTT71160CA