Field Production
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Article (PDF)
Advances in Engineering Research, volume 170 7th International Conference on Energy and Environmental Protection (ICEEP 2018) Effects of water stress on the growth status of five species of shrubs Pingwei Xiang1,a, Xiaoli Ma1,b, Xuefeng Liu1,c, Xiangcheng Yuan1,d, Zhihua Fu2,e* 1Chongqing three gorges academy of agricultural sciences, Wanzhou, Chongqing, China 2Chongqing Three Gorges Vocational College, Wanzhou, Chongqing,China [email protected],[email protected],[email protected], [email protected],[email protected] *Corresponding author. Pingwei Xiang and Xiaoli Ma contributed equally to this work. Keywords: Water stress; shrubs; leaf relative water content; leaf water retention Abstract: A pot experiments were conducted to study the effects of water stress on plant Blade retention, leaf relative moisture content (LRWC), Morphology and growth status, five drought-resistant plants (Pyracantha angustifolia, Pyracantha fortuneana, Pyracantha fortuneana ‘Harlequin’, Ligustrum japonicum ‘Howardii’, Photinia glabra×Photinia serrulata ) were used as materials. The results showed that the drought resistant ability of five species of shrubs from strong to weak as follows: Ligustrum japonicum 'Howardii',Pyracantha fortuneana , Pyracantha angustifolia,Photinia glabra×Photinia serrulata,Pyracantha fortuneana 'Harlequin'. It was found with several Comprehensive indicators that the drought tolerance of Ligustrum japonicum 'Howardii' was significantly higher than that of the other four species, and its adaptability to water deficit was stronger, -

Ships of 8 Tree Species in Navajo National Monument, Arizona
Population Dynamics and Age Relation- ships of 8 Tree Species in Navajo National Monument, Arizona J.D. BROTHERSON, S.R. RUSHFORTH, W.E. EVENSON, J.R. JOHANSEN, AND C. MORDEN The presence of 3 major archeological ruins dating from the 1 lth cent slickrock areas and the plateau behind the canyon rim to 13th centuries (Woodbury 1963) provided the primary motiva- (Brotherson et al. 1978). The pigmy woodland community covers tion for including Navajo National Monument in the National more area than any other type in northeastern Arizona. Parks System. Also included in the monument are some unique Gambel oak is the most extensive type in the Monument aside ecosystems, especially a small relict of “mountain vegetation” from tne pinyon-juniper community. It is found inall 3 segments of found in Betatakin Canyon. As visitor pressures mount annually, the monument but reaches its greatest development at Keet Steel proper management of these unique ecosystems becomes highly and Betatakin. Pnrnus emarginata (bittercherry) also grows at important. Since trees are the dominant features of these ecosys- Keet Steel but is uncommon. tems, and are central to management considerations, the present The streamside community of the Inscription House segment of work has examined populations of 8 major tree species in the the monument contains Popufus fremontii (Fremont poplar), monument. Populus angustifolia (narrowleaf cottonwood), Salk goodingii The objectives of this study were, first, to develop age prediction (Gooding willow), Tamarix ramosissima (salt cedar), and Elaeg- equations for tree species growing in Navajo National Monument, nus angustifolia (Russian olive). Fremont poplar is the dominant and second, to assess the present age profiles, reproductive recruit- tree within this community with salt cedar attaining local impor- ment, and density relationships of these tree populations. -

Phylogeny of Maleae (Rosaceae) Based on Multiple Chloroplast Regions: Implications to Genera Circumscription
Hindawi BioMed Research International Volume 2018, Article ID 7627191, 10 pages https://doi.org/10.1155/2018/7627191 Research Article Phylogeny of Maleae (Rosaceae) Based on Multiple Chloroplast Regions: Implications to Genera Circumscription Jiahui Sun ,1,2 Shuo Shi ,1,2,3 Jinlu Li,1,4 Jing Yu,1 Ling Wang,4 Xueying Yang,5 Ling Guo ,6 and Shiliang Zhou 1,2 1 State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China 2University of the Chinese Academy of Sciences, Beijing 100043, China 3College of Life Science, Hebei Normal University, Shijiazhuang 050024, China 4Te Department of Landscape Architecture, Northeast Forestry University, Harbin 150040, China 5Key Laboratory of Forensic Genetics, Institute of Forensic Science, Ministry of Public Security, Beijing 100038, China 6Beijing Botanical Garden, Beijing 100093, China Correspondence should be addressed to Ling Guo; [email protected] and Shiliang Zhou; [email protected] Received 21 September 2017; Revised 11 December 2017; Accepted 2 January 2018; Published 19 March 2018 Academic Editor: Fengjie Sun Copyright © 2018 Jiahui Sun et al. Tis is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Maleae consists of economically and ecologically important plants. However, there are considerable disputes on generic circumscription due to the lack of a reliable phylogeny at generic level. In this study, molecular phylogeny of 35 generally accepted genera in Maleae is established using 15 chloroplast regions. Gillenia isthemostbasalcladeofMaleae,followedbyKageneckia + Lindleya, Vauquelinia, and a typical radiation clade, the core Maleae, suggesting that the proposal of four subtribes is reasonable. -

Devonian Plant Fossils a Window Into the Past
EPPC 2018 Sponsors Academic Partners PROGRAM & ABSTRACTS ACKNOWLEDGMENTS Scientific Committee: Zhe-kun Zhou Angelica Feurdean Jenny McElwain, Chair Tao Su Walter Finsinger Fraser Mitchell Lutz Kunzmann Graciela Gil Romera Paddy Orr Lisa Boucher Lyudmila Shumilovskikh Geoffrey Clayton Elizabeth Wheeler Walter Finsinger Matthew Parkes Evelyn Kustatscher Eniko Magyari Colin Kelleher Niall W. Paterson Konstantinos Panagiotopoulos Benjamin Bomfleur Benjamin Dietre Convenors: Matthew Pound Fabienne Marret-Davies Marco Vecoli Ulrich Salzmann Havandanda Ombashi Charles Wellman Wolfram M. Kürschner Jiri Kvacek Reed Wicander Heather Pardoe Ruth Stockey Hartmut Jäger Christopher Cleal Dieter Uhl Ellen Stolle Jiri Kvacek Maria Barbacka José Bienvenido Diez Ferrer Borja Cascales-Miñana Hans Kerp Friðgeir Grímsson José B. Diez Patricia Ryberg Christa-Charlotte Hofmann Xin Wang Dimitrios Velitzelos Reinhard Zetter Charilaos Yiotis Peta Hayes Jean Nicolas Haas Joseph D. White Fraser Mitchell Benjamin Dietre Jennifer C. McElwain Jenny McElwain Marie-José Gaillard Paul Kenrick Furong Li Christine Strullu-Derrien Graphic and Website Design: Ralph Fyfe Chris Berry Peter Lang Irina Delusina Margaret E. Collinson Tiiu Koff Andrew C. Scott Linnean Society Award Selection Panel: Elena Severova Barry Lomax Wuu Kuang Soh Carla J. Harper Phillip Jardine Eamon haughey Michael Krings Daniela Festi Amanda Porter Gar Rothwell Keith Bennett Kamila Kwasniewska Cindy V. Looy William Fletcher Claire M. Belcher Alistair Seddon Conference Organization: Jonathan P. Wilson -
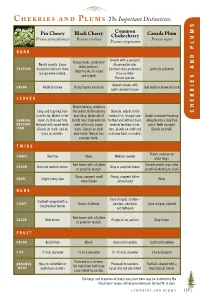
C P the Important Distinctions
C P The Important Distinctions S Common M Pin Cherry Black Cherry Chokecherry Canada Plum Prunus pennsylvanica Prunus serotina Prunus nigra U Prunus virginiana L P BARK D Smooth with a pungent, Young trunks: prominent N Nearly smooth. Large disagreeable odor. white lenticals. TEXTURE horizontal lenticels show Lenticels less prominent Lenticels yellowish A Older trunks: fissured orange when rubbed. than on other and ridged. Prunus species. S E Grayish-brown, with I COLOR Reddish-brown Young trunks are black Dull reddish-brown to black light-colored fissures R LEAVES R E Elliptic/oblong, widest in H Long and tapering from the center, thick leathery Obovate, widest in the C base to tip. Widest in the and shiny. Underside of terminal 1⁄3, sharply saw- Ovate or obovate tapering GENERAL lower 1⁄3; thin and firm midrib near stalk end cov- toothed and without hairs, abruptly into a long thin DESCRIP- textured with round teeth. ered with rusty, brown medium leathery in tex- point. Teeth rounded. TION Glands on stalk, and no hairs. Glands on stalk ture, glands on stalk and Glands on stalk. hairs on midribs. near blade. Margin has no brown hairs on midrib. rounded teeth. TWIGS Thorns common on SHAPE Very fine Waxy Medium slender older twigs Red-brown with a lighter Current growth gray, older COLOR Red and reddish-brown Gray or purplish-brown or greenish margin growth darkening to black Sharp, pungent smell Strong, pungent bitter- ODOR Slight cherry odor None when broken almond odor BUDS Cone shaped, slender – Football-shaped with a SHAPE Ovate, -

TREES for WESTERN NEBRASKA Justin Evertson & Bob Henrickson
THE NEBRASKA STATEWIDE ARBORETUM PRESENTS TREES FOR WESTERN NEBRASKA Justin Evertson & Bob Henrickson. For more plant information, visit plantnebraska.org or retreenbraska.unl.edu The following species are recommended for areas in the western half of Nebraska and/or typically receive less than 20” of moisture per year. Size Range: The size range indicated for each plant is the expected average mature height x spread for Nebraska. Large Deciduous Trees (typically over 40 feet tall at maturity) NOTE ON ASH SPECIES: Native American ash trees are being decimated by Emerald Ash Borer (EAB) and the insect is now in Nebraska. NSA recommends that native ash species no longer be planted in Nebraska. 1. Ash, Manchurian - Fraxinus mandshurica (from Asia; upright growth; drought tolerant; may be resistant to EAB; 40’x 30’) 2. Catalpa, Northern - Catalpa speciosa (native; tough tree; large, heart-shaped leaves, showy flowers and long seed pods; 50’x 35’) 3. Coffeetree, Kentucky - Gymnocladus dioicus (native; amazingly adaptable; beautiful winter form; 50’x 40’) 4. Cottonwood, Eastern - Populus deltoides (majestic native; not for extremely dry sites; avoid most cultivars; 80’x 60’) 5. Cottonwood, Lanceleaf - Populus acuminata (native; naturally occurring hybrid; narrow leaves; for west. G.P.; 50’x 35’) 6. Elm, American - Ulums americana (disease resistant varieties include ‘Valley Forge’ and ‘New Harmony’; 50’x50’) 7. Elm, Japanese - Ulmus davidiana var. japonica (cold tolerant; rounded; glossy green; ‘Discovery’ is a cultivar from Manitoba Canada; 45’x 45’) 8. Elm, Rock - Ulmus thomasii (distinctive corky stems; upright habit; DED resistance in west; 50-60’x 30-40’) New Elm, Hybrids - many disease resistant hybrid elms have been developed and show promise, including: 9. -
![Gether with a Significant Increase in the Lag Phase Preceding Plasma Lipid Oxidation [4]](https://docslib.b-cdn.net/cover/2930/gether-with-a-signi-cant-increase-in-the-lag-phase-preceding-plasma-lipid-oxidation-4-632930.webp)
Gether with a Significant Increase in the Lag Phase Preceding Plasma Lipid Oxidation [4]
Journal of Berry Research 6 (2016) 159–173 159 DOI:10.3233/JBR-160127 IOS Press Multi-radical (ORACMR5) antioxidant capacity of selected berries and effects of food processing R.L. Priora,∗, M. Sintarab and T. Changb aDepartment of Food Science, University of Arkansas, Searcy, AR, USA bInternational Chemical Testing, Milford, MA, USA Received 30 December 2015; accepted 12 March 2016 Abstract. BACKGROUND: Fruits and berries are known to contain relatively high amounts of antioxidant/bioactive compounds. Several methods have been used for measurement of antioxidant capacity (AC), but not all methods have direct relevance to in vivo antioxidant status. OBJECTIVE: Determine AC in berry/fruit samples, processed berry products, and purified compounds by utilizing 5 different biologically relevant free radical/oxidant sources. METHODS: Samples were assayed for AC capacity using 5 different free radical/oxidant sources: peroxyl radical (ORAC), hydroxyl radical (HORAC), peroxynitrite (NORAC), superoxide anion (SORAC) and singlet oxygen (SOAC)]. Total AC (sum of AC with 5 individual radicals) was expressed as Oxygen Radical Absorption Capacity using Multiple Radicals (ORACMR5). RESULTS: SOAC contributed more than 60% of ORACMR5 in blackberries, sweet and tart cherries; and no detectable levels of SOAC were found in strawberries, black currants and raspberries. Whole fruit purees of mango, wild blueberry and cherry contained 95, 85 and 67% respectively of total ORACMR5 from SOAC. However, freeze dried wild blueberry powder from same production season had only 28% as SOAC and 42 and 22% as ORAC and HORAC. Blueberry/Pomegranate and Mango/Pineapple smoothies had 67% and 77% of ORACMR5 as SOAC. CONCLUSIONS: The antioxidant quenching potential using 5 different radical/oxidant sources of different berries and fruits varied widely and understanding this variation may be helpful in understanding health benefits of different berries and foods. -

1. Ground Cover. Botanical Name Asparagus Sprengeri Liex Cornuta Rotunda Juniperus Liriope Pyracantha Walderii 2. Evergreen Tree
Approved Plant List 1. Ground Cover. Botanical name Common Name Asparagus Sprengeri Asparagus Fern Liex Cornuta Rotunda Dwarf Holly Juniperus Various Juniper Ground Liriope Covers Lily Turf Pyracantha Walderii Walders Dwarf Pyracantha 2. Evergreen Trees. Botanical Name Common Name Cinnamomum Camphora Camphor Eriobotrya Japonica Loquat Tree Ligustrum Japonicum Wax Leaf Privet Ligustrum Lucidum Glossy Privet Magnolia Grandiflora Magnolia Magnolia Viginiana Sweet Bay Pinu Elliottiif Slash Pine Slash Pine 3. Palms. Botanical Name Common Name Livistona Chinensis Chinese Fan Palm Butia Capitata Pindo Palm Chamaerops Humilis European Fan Palm Sabal Palmetto Cabbage Palm Phoenix Robenimum Pigmy Date Palm Washingtonia Robusta Mexican Fan Falm Cycas Revoluta Sa 4. Shrubs. Botanical Name Common Name Raphiolepsis Indica Indian Hawthorne Cocculus Laurifolius Snail seed go Palm Cortaderia Selloana Pampas Grass Eleagnus Pungens Silverthorn Llex Burfordii Burford Holly Llex Vomitoria Yaupon Holly Juniperus Spp. Various Juniper Shrubs Glossy Ligustrum Lucidum Privet Mahonia Bealei Leatherleaf Mahonia Myrica Cerifera Wax Myrtle Nandina Domestica Heavenly Bamboo Amelia Walk – A Planned Community Architectural Planning Criteria – 4th Revision – December 22, 2015 Page | 16 Photinia Glabra Red Photinia Pittosporum Spp. Various Pittosporums Pyracantha Coccinea Firethorn Trachelospermum Jasminoides Confederate Jasmine Viburmum Odoratissimum Sweet Viburnum Viburnum Suspensum Sandankwa Viburnum Ilex crenata 'Compacta’ Compacta Holly 5. Shade Trees. Botanical Name Common Name Quercus Virginiana Live Oak Quercus Laurifolia Laurel Oak Acer Rubrum Red Maple Betula Nigra River Birch Cornus Dogwood 6. Ornamental Trees. Botanical Name Common Name Pyrus Calleryiana Bradford Pear Photinia Fraseri Tree Photinia (Red Tip) Ilex x ‘Nellie R. Stevens’ Nellie Stevens Holly Lagerstroemia Crape Myrtle Amelia Walk – A Planned Community Architectural Planning Criteria – 4th Revision – December 22, 2015 Page | 17 . -

(Rosaceae), I. Differentiation of Mespilus and Crataegus
Phytotaxa 257 (3): 201–229 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2016 Magnolia Press Article ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.257.3.1 STUDIES IN MESPILUS, CRATAEGUS, AND ×CRATAEMESPILUS (ROSACEAE), I. DIFFERENTIATION OF MESPILUS AND CRATAEGUS, EXPANSION OF ×CRATAEMESPILUS, WITH SUPPLEMENTARY OBSERVATIONS ON DIFFERENCES BETWEEN THE CRATAEGUS AND AMELANCHIER CLADES JAMES B. PHIPPS Department of Biology, The University of Western Ontario, London, Ontario N6A 5B7, Canada; email: [email protected] Abstract The paper argues the position for retaining a monotypic Mespilus, i.e., in the sense of M. germanica, the medlar. Recent cladistic papers lend support for Mespilus being sister to Crataegus, and there is a clear morphological distinction from Cra- taegus, emphasized by adaptation to carnivore frugivory. Mespilus secured, the paper then treats each of the known hybrids between Mespilus and Crataegus, making the new combination Crataemespilus ×canescens (J.B. Phipps) J.B. Phipps. Keywords: Crataemespilus ×canescens (J.B. Phipps) J.B. Phipps comb. nov.; inflorescence position; medlar; Mespilus a folk-genus; Mespilus distinct from Crataegus; Rosaceae; taxonomic history of Mespilus Introduction The author has a long-standing interest in generic delimitation in the Maloid genera of the Rosaceae (Maleae Small, formerly Maloideae C. Weber, Pyrinae Dumort.), as shown particularly in a series of papers with K. Robertson, J. Rohrer, and P.G. Smith (Phipps et al. 1990, 1991; Robertson at al. 1991, 1992; Rohrer at al. 1991, 1994) which treated all 28 genera of Maleae as recognised by us. There is also a revisionary treatment of New World Heteromeles M.J. -

The Structure and Composition of Vegetation in the Lake-Fill Peatlands of Indiana
2001. Proceedings of the Indiana Academy of Science 1 10:51-78 THE STRUCTURE AND COMPOSITION OF VEGETATION IN THE LAKE-FILL PEATLANDS OF INDIANA Anthony L. Swinehart 1 and George R. Parker: Department of Forestry and Natural Resources, Purdue University, West Lafayette, Indiana 47907 Daniel E. Wujek: Department of Biology, Central Michigan University, Mount Pleasant, Michigan 48859 ABSTRACT. The vegetation of 16 lake-fill peatlands in northern Indiana was systematically sampled. Peatland types included fens, tall shrub bogs, leatherleaf bogs and forested peatlands. No significant difference in species richness among the four peatland types was identified from the systematic sampling. Vegetation composition and structure, along with water chemistry variables, was analyzed using multi- variate statistical analysis. Alkalinity and woody plant cover accounted for much of the variability in the herbaceous and ground layers of the peatlands, and a successional gradient separating the peatlands was evident. A multivariate statistical comparison of leatherleaf bogs from Indiana, Michigan, Ohio, New York, New Jersey and New Hampshire was made on the basis of vegetation composition and frequency and five climatic variables. The vascular vegetation communities of Indiana peatlands and other peatlands in the southern Great Lakes region are distinct from those in the northeastern U.S., Ohio and the northern Great Lakes. Some of these distinctions are attributed to climatic factors, while others are related to biogeo- graphic history of the respective regions. Keywords: Peatlands, leatherleaf bogs, fens, ecological succession, phytogeography Within midwestern North America, the such as Chamaedaphne calyculata, Androm- northern counties of Illinois, Indiana and Ohio eda glaucophylla, and Carex oligospermia of- 1 represent the southern extent of peatland com- ten make "southern outlier peatlands ' con- munities containing characteristic plant spe- spicuous to botanists, studies of such cies of northern or boreal affinity. -

Mysterious Chokeberries: New Data on the Diversity and Phylogeny of Aronia Medik. (Rosaceae)
European Journal of Taxonomy 570: 1–14 ISSN 2118-9773 https://doi.org/10.5852/ejt.2019.570 www.europeanjournaloftaxonomy.eu 2019 · Shipunov A. et al. This work is licensed under a Creative Commons Attribution License (CC BY 4.0). Research article Mysterious chokeberries: new data on the diversity and phylogeny of Aronia Medik. (Rosaceae) Alexey SHIPUNOV 1,*, Sofia GLADKOVA 2, Polina TIMOSHINA 3, Hye Ji LEE 4, Jinhee CHOI 5, Sarah DESPIEGELAERE 5 & Bryan CONNOLLY 5 1,4,5,6 Minot State University, Biology, 500 University Ave, Minot, ND, USA. 2,3 Department of Biology, Moscow State University, Russia. 7 Framingham State University, Biology, 100 State St, Framingham, MA, USA. * Corresponding author: [email protected] 2 Email: [email protected] 3 Email: [email protected] 4 Email: [email protected] 5 Email: [email protected] 6 Email: [email protected] 7 Email: [email protected] Abstract. Aronia Medik. (chokeberry, Rosaceae) is a genus of woody shrubs with two or three North American species. Species boundaries and relationships between species of Aronia are frequently under question. The only European species in the genus, A. mitschurinii A.K.Skvortsov & Maitul., is suggested to be an inter-generic hybrid. In order to clarify the relationships between species of Aronia, we performed several morphometric and molecular analyses and found that the molecular and morphological diversity within data on American Aronia is low, and species boundaries are mostly not clearly expressed. Whereas morphology is able to separate American species from A. mitschurinii, there is no support for such discrimination from the molecular data; our analyses did not reveal evidence of A. -

Chokecherry Prunus Virginiana L
chokecherry Prunus virginiana L. Synonyms: Prunus virginiana ssp. demissa (Nutt.) Roy L. Taylor & MacBryde, P. demissa (Nutt.) Walp. Other common names: black chokecherry, bitter-berry, cabinet cherry, California chokecherry, caupulin, chuckleyplum, common chokecherry, eastern chokecherry, jamcherry, red chokecherry, rum chokecherry, sloe tree, Virginia chokecherry, western chokecherry, whiskey chokecherry, wild blackcherry, wild cherry Family: Rosaceae Invasiveness Rank: 74 The invasiveness rank is calculated based on a species’ ecological impacts, biological attributes, distribution, and response to control measures. The ranks are scaled from 0 to 100, with 0 representing a plant that poses no threat to native ecosystems and 100 representing a plant that poses a major threat to native ecosystems. Description Similar species: Several non-native Prunus species can Chokecherry is a deciduous, thicket-forming, erect shrub be confused with chokecherry in Alaska. Unlike or small tree that grows 1 to 6 m tall from an extensive chokecherry, which has glabrous inner surfaces of the network of lateral roots. Roots can extend more than 10 basal sections of the flowers, European bird cherry m horizontally and 2 m vertically. Young twigs are often (Prunus padus) has hairy inner surfaces of the basal hairy. Stems are numerous, slender, and branched. Bark sections of the flowers. Chokecherry can also be is smooth to fine-scaly and red-brown to grey-brown. differentiated from European bird cherry by its foliage, Leaves are alternate, elliptic to ovate, and 3 to 10 cm which turns red in late summer and fall; the leaves of long with pointed tips and toothed margins. Upper European bird cherry remain green throughout the surfaces are green and glabrous, and lower surfaces are summer.