Anthropoid Primates from the Oligocene of Pakistan (Bugti Hills): Data on Early Anthropoid Evolution and Biogeography
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

Fascinating Primates 3/4/13 8:09 AM Ancient Egyptians Used Traits of an Ibis Or a Hamadryas Used Traits Egyptians Ancient ) to Represent Their God Thoth
© Copyright, Princeton University Press. No part of this book may be distributed, posted, or reproduced in any form by digital or mechanical means without prior written permission of the publisher. Fascinating Primates Fascinating The Beginning of an Adventure Ever since the time of the fi rst civilizations, nonhuman primates and people have oc- cupied overlapping habitats, and it is easy to imagine how important these fi rst contacts were for our ancestors’ philosophical refl ections. Long ago, adopting a quasi- scientifi c view, some people accordingly regarded pri- mates as transformed humans. Others, by contrast, respected them as distinct be- ings, seen either as bearers of sacred properties or, conversely, as diabolical creatures. A Rapid Tour around the World In Egypt under the pharaohs, science and religion were still incompletely separated. Priests saw the Papio hamadryas living around them as “brother baboons” guarding their temples. In fact, the Egyptian god Thoth was a complex deity combining qualities of monkeys and those of other wild animal species living in rice paddies next to temples, all able to sound the alarm if thieves were skulking nearby. At fi rst, baboons represented a local god in the Nile delta who guarded sacred sites. The associated cult then spread through middle Egypt. Even- tually, this god was assimilated by the Greeks into Hermes Trismegistus, the deity measuring and interpreting time, the messenger of the gods. One conse- quence of this deifi cation was that many animals were mummifi ed after death to honor them. Ancient Egyptians used traits of an ibis or a Hamadryas Baboon (Papio hamadryas) to represent their god Thoth. -

8. Primate Evolution
8. Primate Evolution Jonathan M. G. Perry, Ph.D., The Johns Hopkins University School of Medicine Stephanie L. Canington, B.A., The Johns Hopkins University School of Medicine Learning Objectives • Understand the major trends in primate evolution from the origin of primates to the origin of our own species • Learn about primate adaptations and how they characterize major primate groups • Discuss the kinds of evidence that anthropologists use to find out how extinct primates are related to each other and to living primates • Recognize how the changing geography and climate of Earth have influenced where and when primates have thrived or gone extinct The first fifty million years of primate evolution was a series of adaptive radiations leading to the diversification of the earliest lemurs, monkeys, and apes. The primate story begins in the canopy and understory of conifer-dominated forests, with our small, furtive ancestors subsisting at night, beneath the notice of day-active dinosaurs. From the archaic plesiadapiforms (archaic primates) to the earliest groups of true primates (euprimates), the origin of our own order is characterized by the struggle for new food sources and microhabitats in the arboreal setting. Climate change forced major extinctions as the northern continents became increasingly dry, cold, and seasonal and as tropical rainforests gave way to deciduous forests, woodlands, and eventually grasslands. Lemurs, lorises, and tarsiers—once diverse groups containing many species—became rare, except for lemurs in Madagascar where there were no anthropoid competitors and perhaps few predators. Meanwhile, anthropoids (monkeys and apes) emerged in the Old World, then dispersed across parts of the northern hemisphere, Africa, and ultimately South America. -

Fossil Primates
AccessScience from McGraw-Hill Education Page 1 of 16 www.accessscience.com Fossil primates Contributed by: Eric Delson Publication year: 2014 Extinct members of the order of mammals to which humans belong. All current classifications divide the living primates into two major groups (suborders): the Strepsirhini or “lower” primates (lemurs, lorises, and bushbabies) and the Haplorhini or “higher” primates [tarsiers and anthropoids (New and Old World monkeys, greater and lesser apes, and humans)]. Some fossil groups (omomyiforms and adapiforms) can be placed with or near these two extant groupings; however, there is contention whether the Plesiadapiformes represent the earliest relatives of primates and are best placed within the order (as here) or outside it. See also: FOSSIL; MAMMALIA; PHYLOGENY; PHYSICAL ANTHROPOLOGY; PRIMATES. Vast evidence suggests that the order Primates is a monophyletic group, that is, the primates have a common genetic origin. Although several peculiarities of the primate bauplan (body plan) appear to be inherited from an inferred common ancestor, it seems that the order as a whole is characterized by showing a variety of parallel adaptations in different groups to a predominantly arboreal lifestyle, including anatomical and behavioral complexes related to improved grasping and manipulative capacities, a variety of locomotor styles, and enlargement of the higher centers of the brain. Among the extant primates, the lower primates more closely resemble forms that evolved relatively early in the history of the order, whereas the higher primates represent a group that evolved more recently (Fig. 1). A classification of the primates, as accepted here, appears above. Early primates The earliest primates are placed in their own semiorder, Plesiadapiformes (as contrasted with the semiorder Euprimates for all living forms), because they have no direct evolutionary links with, and bear few adaptive resemblances to, any group of living primates. -

Early Eocene Primates from Gujarat, India
ARTICLE IN PRESS Journal of Human Evolution xxx (2009) 1–39 Contents lists available at ScienceDirect Journal of Human Evolution journal homepage: www.elsevier.com/locate/jhevol Early Eocene Primates from Gujarat, India Kenneth D. Rose a,*, Rajendra S. Rana b, Ashok Sahni c, Kishor Kumar d, Pieter Missiaen e, Lachham Singh b, Thierry Smith f a Johns Hopkins University School of Medicine, Baltimore, Maryland 21205, USA b H.N.B. Garhwal University, Srinagar 246175, Uttarakhand, India c Panjab University, Chandigarh 160014, India d Wadia Institute of Himalayan Geology, Dehradun 248001, Uttarakhand, India e University of Ghent, B-9000 Ghent, Belgium f Royal Belgian Institute of Natural Sciences, B-1000 Brussels, Belgium article info abstract Article history: The oldest euprimates known from India come from the Early Eocene Cambay Formation at Vastan Mine Received 24 June 2008 in Gujarat. An Ypresian (early Cuisian) age of w53 Ma (based on foraminifera) indicates that these Accepted 8 January 2009 primates were roughly contemporary with, or perhaps predated, the India-Asia collision. Here we present new euprimate fossils from Vastan Mine, including teeth, jaws, and referred postcrania of the Keywords: adapoids Marcgodinotius indicus and Asiadapis cambayensis. They are placed in the new subfamily Eocene Asiadapinae (family Notharctidae), which is most similar to primitive European Cercamoniinae such as India Donrussellia and Protoadapis. Asiadapines were small primates in the size range of extant smaller Notharctidae Adapoidea bushbabies. Despite their generally very plesiomorphic morphology, asiadapines also share a few derived Omomyidae dental traits with sivaladapids, suggesting a possible relationship to these endemic Asian adapoids. In Eosimiidae addition to the adapoids, a new species of the omomyid Vastanomys is described. -

Evidence for an Asian Origin of Stem Anthropoids
Evidence for an Asian origin of stem anthropoids Richard F. Kay1 Department of Evolutionary Anthropology, Duke University, Durham, NC 27708-03083 n PNAS, Chaimanee et al. (1) report Genetic, embryological, and anatomical We are not there yet. Recent comprehen- a previously undescribed species of evidence demonstrates that the sister sive phylogenetic analyses stemming from I primate, Afrasia, from the late Middle group of Anthropoidea is the south Asian virtually the same datasets yield somewhat Eocene of Burma. They identify tarsier, with the two forming the crown different cladograms that reflect sensitivity Afrasia as the sister taxon to the African group Haplorhini (6–8). The other clade of to which taxa are included in the analysis genus Afrotarsius but slightly more primitive extant primates is the lemurs and lorises, and which sets of analytical assumptions are than it and allied with stem Anthropoidea called Strepsirrhini. Eocene Holarctic selected. Pertinent to the biogeographic of south Asia. Anthropoidea is the taxo- Omomyoidea are generally considered as conclusions of Chaimanee et al. (1), Seiffert nomic group that today includes New and stem haplorhines, although the precise et al. (11) conclude that Afrotarsius cha- Old World monkeys, apes, and humans. If relationship of omomyoids to tarsiers and trathi [a younger species than the one that upheld, the biogeographic significance of anthropoids is uncertain (8). Tarsius often Chaimanee et al. (1) describe] is an African these results is profound: If Afrasia and is considered to be a relictual omomyoid tarsioid. If Seiffert et al. (11) are correct, Afrotarsius are as closely related as Chai- in south Asia. -

Effects of Additional Postcranial Data on the Topologies of Early Eocene Primate
Effects of Additional Postcranial Data on the Topologies of Early Eocene Primate Phylogenies Julia Stone ANT 679HB Special Honors in the Department of Anthropology The University of Texas at Austin May 2021 Liza J. Shapiro Department of Anthropology Supervising Professor E. Christopher Kirk Department of Anthropology Second Reader Acknowledgements This project would not have been completed without the help of my thesis supervisor Dr. Liza Shapiro. I was inspired to start the project after taking her courses in Primate Evolution and Primate Anatomy. She also met with me many times throughout this year to discuss the trajectory of the project as well as discuss papers that became the foundation of the thesis. She assisted me through technology issues, unresolved trees, and many drafts, and she provided invaluable guidance throughout this process. I also want to thank Dr. Christopher Kirk for being my second reader and providing extremely helpful insight into how to write about phylogenetic results. He also helped me to be more specific and accurate in all of the sections of the thesis. I learned a lot about writing theses in general due to his comments on my drafts. I also truly appreciate the help of Ben Rodwell, a graduate student at the University of Texas at Austin. He provided excellent help and support regarding the Mesquite and TNT software used in this project. ii Effects of Additional Postcranial Data on the Topologies of Early Eocene Primate Phylogenies by Julia Stone, BA The University of Texas at Austin SUPERVISOR: Liza J. Shapiro There are multiple hypotheses regarding the locomotor behaviors of the last common ancestor to primates and which fossil primates best represent that ancestor. -
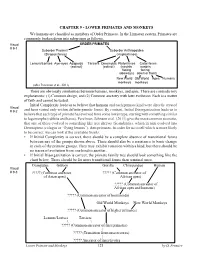
CHAPTER 9 - LOWER PRIMATES and MONKEYS We Humans Are Classified As Members of Order Primates
CHAPTER 9 - LOWER PRIMATES AND MONKEYS We humans are classified as members of Order Primates. In the Linnaean system, Primates are commonly broken down into subgroups as follows. Visual ORDER PRIMATES # 9-1 Suborder Prosimii Suborder Anthropoidea (Strepsirrhines) (Haplorrhines) Lemurs/Lorises Aye-ayes Adapoids Tarsiers Omomyids Platyrrhines Catarrhines (extinct) (extinct) (nostrils nostrils facing facing sideways) down or front) New World Old World Apes Humans monkeys monkeys (after Perelman et al., 2011) There are obviously similarities between humans, monkeys, and apes. There are contradictory explanations: (1) Common design, and (2) Common ancestry with later evolution. Each is a matter of faith and cannot be tested. Initial Complexity leads us to believe that humans and each primate kind were directly created Visual # 9-2 and have varied only within definite genetic limits. By contrast, Initial Disorganization leads us to believe that each type of primate has evolved from some lower type, starting with something similar to lagomorphs (rabbits and hares). Perelman, Johnson et al. (2011) give the most common scenario, that one of these evolved to something like tree shrews (Scandentia), which in turn evolved into Dermoptera (colugos or “flying lemurs”), then primates. In order for us to tell which is more likely to be correct, we can look at the available fossils. • If Initial Complexity is correct, there should be a complete absence of transitional forms between any of the groups shown above. There should also be a resistance to basic change in each of the primate groups. They may exhibit variation within a kind, but there should be no traces of evolution from one kind to another. -

(Tarsius Pumilus) in CENTRAL SULAWESI, INDONESIA
ALTITUDINAL EFFECTS ON THE BEHAVIOR AND MORPHOLOGY OF PYGMY TARSIERS (Tarsius pumilus) IN CENTRAL SULAWESI, INDONESIA A Dissertation by NANDA BESS GROW Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Chair of Committee, Sharon Gursky-Doyen Committee Members, Michael Alvard Jeffrey Winking Jane Packard Head of Department, Cynthia Werner August 2013 Major Subject: Anthropology Copyright 2013 Nanda Bess Grow ABSTRACT Pygmy tarsiers (Tarsius pumilus) of Central Sulawesi, Indonesia are the only species of tarsier known to live exclusively at high altitudes. This study was the first to locate and observe multiple groups of this elusive primate. This research tested the hypothesis that variation in pygmy tarsier behavior and morphology correlates with measurable ecological differences that occur along an altitudinal gradient. As a response to decreased resources at higher altitudes and the associated effects on foraging competition and energy intake, pygmy tarsiers were predicted to exhibit lower population density, smaller group sizes, larger home ranges, and reduced sexually selected traits compared to lowland tarsiers. Six groups containing a total of 22 individuals were observed. Pygmy tarsiers were only found between 2000 and 2300 m, indicating allopatric separation from lowland tarsiers. As expected, the observed pygmy tarsiers lived at a lower density than lowland tarsier species, in association with decreased resources at higher altitudes. The estimated population density of pygmy tarsiers was 92 individuals per 100 ha, with 25 groups per 100 ha. However, contrary to expectation, home range sizes were not significantly larger than lowland tarsier home ranges, and average NPL was smaller than those of lowland tarsiers. -

The Tempo of Trophic Evolution in Small-Bodied Primates 2 3 4 Running Title: Trophic Evolution in Small-Bodied Primates 5 6 7 Jeremiah E
bioRxiv preprint doi: https://doi.org/10.1101/2020.03.17.996207; this version posted March 20, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. 1 1 The tempo of trophic evolution in small-bodied primates 2 3 4 Running title: Trophic evolution in small-bodied primates 5 6 7 Jeremiah E. Scott 8 Department of Medical Anatomical Sciences 9 College of Osteopathic Medicine of the Pacific 10 Western University of Health Sciences 11 309 East Second Street 12 Pomona, California 91766 13 U.S.A. 14 [email protected] 15 16 17 Abstract 18 19 Objectives: As a primary trophic strategy, insectivory is uncommon and unevenly 20 distributed across extant primates. This pattern is partly a function of the challenges that 21 insectivory poses for large-bodied primates. In this study, I demonstrate that the uneven 22 distribution is also a consequence of variation in the rate of trophic evolution among small- 23 bodied lineages. 24 Methods: The sample consisted of 307 species classified by primary trophic strategy and 25 body size, creating an ordered three-state character: small-insectivorous, small-herbivorous, 26 and large-herbivorous. I tested for rate heterogeneity by partitioning major clades from the 27 rest of the primate tree and estimating separate rates of transition between herbivory and 28 insectivory for small-bodied lineages in each partition. 29 Results: Bayesian analysis of rate estimates indicates that a model with two rates of trophic 30 evolution provides the best fit to the data. -

Workflow-Simplified Ancestral State Reconstruction of Discrete Traits with Mrbayes in the R Environment
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.10.426107; this version posted January 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. MBASR: Workflow-simplified ancestral state reconstruction of discrete traits with MrBayes in the R environment 1,2 Steven Heritage 1 Duke Lemur Center Museum of Natural History, Duke University, Durham, NC, United States 2 Interdepartmental Doctoral Program in Anthropological Science, Stony Brook University, Stony Brook, NY, United States Abstract Ancestral state reconstruction (ASR) is a critical tool in comparative biology. Given a phylogenetic tree and observed trait states for taxa at the tree’s tips, ASR aims to model ancestral trait states at the tree’s internal nodes. Thus, the pattern, tempo, and frequency of a character’s evolution can be considered in a phylogenetic framework. Software programs that perform discrete trait ASR analyses are variably complex in terms of workflow steps, input formats, user interfaces, settings and execution commands, and the organization of output. MBASR (acronym for MrBayes Ancestral States with R) is an R language toolkit that highly automates the ASR workflow and uses the machinery of the popular phylogenetics software MrBayes. Input data files are simple, analysis can be setup and run with a single R function, and statistical output is concisely organized and immediately interpretable. Use requires minimal knowledge of the most basic R commands and no experience with the MrBayes program. -

Revised Age Estimates for the Later Paleogene Mammal Faunas of Egypt and Oman
Revised age estimates for the later Paleogene mammal faunas of Egypt and Oman Erik R. Seiffert* Department of Earth Sciences and Museum of Natural History, University of Oxford, Parks Road, Oxford OX1 3PW, United Kingdom Communicated by Elwyn L. Simons, Duke University, Durham, NC, January 30, 2006 (received for review January 3, 2006) The Jebel Qatrani Formation of northern Egypt has produced available from the Formation itself is the magnetostratigraphy Afro-Arabia’s primary record of Paleogene mammalian evolution, developed by Kappelman et al. (17) (Fig. 1A), but there remain including the world’s most complete remains of early anthropoid multiple possible correlations of that magnetostratigraphy to the primates. Recent studies of Fayum mammals have assumed that geomagnetic polarity time scale (GPTS). the Jebel Qatrani Formation contains a significant Eocene compo- The Jebel Qatrani Formation is divided into upper and lower nent (Ϸ150 of 340 m), and that most taxa from that succession are sequences (15), with the boundary between these units being the between 35.4 and 33.3 million years old (Ma), i.e., latest Eocene to 4- to 10-m-thick cliff-forming ‘‘Barite Sandstone’’ that uncon- earliest Oligocene in age. Reanalysis of the chronological evidence formably overlies the upper red sandstone of the lower sequence. shared by later Paleogene strata exposed in Egypt and Oman Rasmussen et al. (16) suggested that the approximate position of (Taqah and Thaytiniti areas, Dhofar Province) reveals that this the EOB is probably marked by this unconformity,