Genome Wide Mapping of Fruit Quality Traits in Apple
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Apples Catalogue 2019
ADAMS PEARMAIN Herefordshire, England 1862 Oct 15 Nov Mar 14 Adams Pearmain is a an old-fashioned late dessert apple, one of the most popular varieties in Victorian England. It has an attractive 'pearmain' shape. This is a fairly dry apple - which is perhaps not regarded as a desirable attribute today. In spite of this it is actually a very enjoyable apple, with a rich aromatic flavour which in apple terms is usually described as Although it had 'shelf appeal' for the Victorian housewife, its autumnal colouring is probably too subdued to compete with the bright young things of the modern supermarket shelves. Perhaps this is part of its appeal; it recalls a bygone era where subtlety of flavour was appreciated - a lovely apple to savour in front of an open fire on a cold winter's day. Tree hardy. Does will in all soils, even clay. AERLIE RED FLESH (Hidden Rose, Mountain Rose) California 1930’s 19 20 20 Cook Oct 20 15 An amazing red fleshed apple, discovered in Aerlie, Oregon, which may be the best of all red fleshed varieties and indeed would be an outstandingly delicious apple no matter what color the flesh is. A choice seedling, Aerlie Red Flesh has a beautiful yellow skin with pale whitish dots, but it is inside that it excels. Deep rose red flesh, juicy, crisp, hard, sugary and richly flavored, ripening late (October) and keeping throughout the winter. The late Conrad Gemmer, an astute observer of apples with 500 varieties in his collection, rated Hidden Rose an outstanding variety of top quality. -

APPLE (Fruit Varieties)
E TG/14/9 ORIGINAL: English DATE: 2005-04-06 INTERNATIONAL UNION FOR THE PROTECTION OF NEW VARIETIES OF PLANTS GENEVA * APPLE (Fruit Varieties) UPOV Code: MALUS_DOM (Malus domestica Borkh.) GUIDELINES FOR THE CONDUCT OF TESTS FOR DISTINCTNESS, UNIFORMITY AND STABILITY Alternative Names:* Botanical name English French German Spanish Malus domestica Apple Pommier Apfel Manzano Borkh. The purpose of these guidelines (“Test Guidelines”) is to elaborate the principles contained in the General Introduction (document TG/1/3), and its associated TGP documents, into detailed practical guidance for the harmonized examination of distinctness, uniformity and stability (DUS) and, in particular, to identify appropriate characteristics for the examination of DUS and production of harmonized variety descriptions. ASSOCIATED DOCUMENTS These Test Guidelines should be read in conjunction with the General Introduction and its associated TGP documents. Other associated UPOV documents: TG/163/3 Apple Rootstocks TG/192/1 Ornamental Apple * These names were correct at the time of the introduction of these Test Guidelines but may be revised or updated. [Readers are advised to consult the UPOV Code, which can be found on the UPOV Website (www.upov.int), for the latest information.] i:\orgupov\shared\tg\applefru\tg 14 9 e.doc TG/14/9 Apple, 2005-04-06 - 2 - TABLE OF CONTENTS PAGE 1. SUBJECT OF THESE TEST GUIDELINES..................................................................................................3 2. MATERIAL REQUIRED ...............................................................................................................................3 -
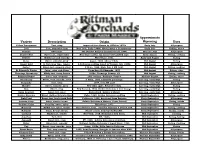
Variety Description Origin Approximate Ripening Uses
Approximate Variety Description Origin Ripening Uses Yellow Transparent Tart, crisp Imported from Russia by USDA in 1870s Early July All-purpose Lodi Tart, somewhat firm New York, Early 1900s. Montgomery x Transparent. Early July Baking, sauce Pristine Sweet-tart PRI (Purdue Rutgers Illinois) release, 1994. Mid-late July All-purpose Dandee Red Sweet-tart, semi-tender New Ohio variety. An improved PaulaRed type. Early August Eating, cooking Redfree Mildly tart and crunchy PRI release, 1981. Early-mid August Eating Sansa Sweet, crunchy, juicy Japan, 1988. Akane x Gala. Mid August Eating Ginger Gold G. Delicious type, tangier G Delicious seedling found in Virginia, late 1960s. Mid August All-purpose Zestar! Sweet-tart, crunchy, juicy U Minn, 1999. State Fair x MN 1691. Mid August Eating, cooking St Edmund's Pippin Juicy, crisp, rich flavor From Bury St Edmunds, 1870. Mid August Eating, cider Chenango Strawberry Mildly tart, berry flavors 1850s, Chenango County, NY Mid August Eating, cooking Summer Rambo Juicy, tart, aromatic 16th century, Rambure, France. Mid-late August Eating, sauce Honeycrisp Sweet, very crunchy, juicy U Minn, 1991. Unknown parentage. Late Aug.-early Sept. Eating Burgundy Tart, crisp 1974, from NY state Late Aug.-early Sept. All-purpose Blondee Sweet, crunchy, juicy New Ohio apple. Related to Gala. Late Aug.-early Sept. Eating Gala Sweet, crisp New Zealand, 1934. Golden Delicious x Cox Orange. Late Aug.-early Sept. Eating Swiss Gourmet Sweet-tart, juicy Switzerland. Golden x Idared. Late Aug.-early Sept. All-purpose Golden Supreme Sweet, Golden Delcious type Idaho, 1960. Golden Delicious seedling Early September Eating, cooking Pink Pearl Sweet-tart, bright pink flesh California, 1944, developed from Surprise Early September All-purpose Autumn Crisp Juicy, slow to brown Golden Delicious x Monroe. -

Germplasm Sets and Standardized Phenotyping Protocols for Fruit Quality Traits in Rosbreed
Germplasm Sets and Standardized Phenotyping Protocols for Fruit Quality Traits in RosBREED Jim Luby, Breeding Team Leader Outline of Presentation RosBREED Demonstration Breeding Programs Standardized Phenotyping Protocols Reference Germplasm Sets SNP Detection Panels Crop Reference Set Breeding Pedigree Set RosBREED Demonstration Breeding Programs Clemson U WSU Texas A&M UC Davis U Minn U Arkansas Rosaceae Cornell U WSU MSU MSU Phenotyping Affiliates USDA-ARS Driscolls Corvallis Univ of Florida UNH Standardized Phenotyping Protocols Traits and Standardized Phenotyping Protocols • Identify critical fruit quality traits and other important traits • Develop standardized phenotyping protocols to enable data pooling across locations/institutions • Protocols available at www.RosBREED.org Apple Standardized Phenotyping Firmness, Crispness – Instrumental, Sensory Sweetness, Acidity – Intstrumental, Sensory Color, Appearance, Juiciness, Aroma – Sensory At harvest Cracking, Russet, Sunburn Storage 10w+7d Storage 20w+7d Maturity Fruit size 5 fruit (reps) per evaluation Postharvest disorders Harvest date, Crop, Dropping RosBREED Apple Phenotyping Locations Wenatchee, WA St Paul, MN Geneva, NY • One location for all evaluations would reduce variation among instruments and evaluators • Local evaluations more sustainable and relevant for future efforts at each institution • Conduct standardized phenotyping of Germplasm Sets at respective sites over multiple (2-3) seasons • Collate data in PBA format, conduct quality control, archive Reference -

Bristol Naturalist News
Contents / Diary of events NOVEMBER 2017 Bristol Naturalist News Discover Your Natural World Bristol Naturalists’ Society BULLETIN NO. 565 NOVEMBER 2017 BULLETIN NO. 565 NOVEMBER 2017 Bristol Naturalists’ Society Discover Your Natural World Registered Charity No: 235494 www.bristolnats.org.uk HON. PRESIDENT: Andrew Radford, Professor CONTENTS of Behavioural Ecology, Bristol University 3 Diary of Events ACTING CHAIRMAN: Stephen Fay HON. PROCEEDINGS RECEIVING EDITOR: 4 Society Walk / Society Talk Dee Holladay, 15 Lower Linden Rd., Clevedon, 5 Lesley’s “Natty News…” BS21 7SU [email protected] HON. SEC.: Lesley Cox 07786 437 528 6 Get Published! Write for Nature in Avon [email protected] HON. MEMBERSHIP SEC: Mrs. Margaret Fay 7 Joint BNS/University programme 81 Cumberland Rd., BS1 6UG. 0117 921 4280 8 Phenology ; Book Club [email protected] Welcome to new members HON. TREASURER: Michael Butterfield 14 Southdown Road, Bristol, BS9 3NL 9 Society Walk Report; (0117) 909 2503 [email protected] Poem for the month BULLETIN DISTRIBUTION 10 BOTANY SECTION Hand deliveries save about £800 a year, so help Botanical notes; Meeting Report; is much appreciated. Offers please to: Plant Records HON. CIRCULATION SEC.: Brian Frost, 60 Purdy Court, New Station Rd, Fishponds, Bristol, BS16 13 GEOLOGY SECTION 3RT. 0117 9651242. [email protected] He will be pleased to supply further details. Also 14 INVERTEBRATE SECTION Notes for this month contact him about problems with (non-)delivery. BULLETIN COPY DEADLINE: 7th of month before 15 LIBRARY Hand-coloured books publication to the editor: David B Davies, The Summer House, 51a Dial Hill Rd., Clevedon, 17 ORNITHOLOGY SECTION BS21 7EW. -

New Apple Cultivars with Resistance to Multiple Diseases on the Way
NEW APPLE CULTIVARS WITH RESISTANCE TO MULTIPLE DISEASES ON THE WAY New apple cultivar development is well underway for RosBREED, thanks to a cutting-edge rapid breeding technique and application of DNA information generated in the project. Dr. Jay Norelli at the USDA-ARS Appalachian Fruit Research Station, Kearneysville, WV, is breeding apple trees with genetic resistance to scab and fire blight, two major challenges for U.S. apple growers. Apple scab is a common fungal disease managed by pesticide sprays and clearing leaf debris at season’s end. Fire blight is a bacterial disease difficult to prevent and even harder to control once an orchard is infected. Fortunately, we have sources of genetic resistance to the two diseases for breeders to use as parents. ‘Enterprise’ carries genetic resistance to both scab (Rvi6 resistance factor originally from the wild species Malus floribunda) and fire blight (FB7 source from heritage cultivar Cox’s Orange Pippin), and ‘Splendour’ carries resistance to fire blight at another genetic location (FB5, original source still under investigation). ‘Honeycrisp’ carries another resistance factor for scab (RviHC) and an allele for its characteristic crisp fruit texture, for which RosBREED has developed a DNA test (Ma-Indel). Using traditional selective breeding strategies to combine all these resistance alleles into a single Norelli’s breeding scheme for combining scab resistance, fire blight individual would take decades, even enhanced with resistance, and fruit quality traits into one apple cultivar. DNA tests. Apple trees generally don’t bloom until they’re around four years old. Even if a breeder knows a young tree would make a good parent, they must wait years to cross it. -

Open KON Dissertation FINAL.Pdf
The Pennsylvania State University The Graduate School College of Agricultural Sciences EVALUATION OF CHEMICAL AND THERMAL BLOSSOM THINNING STRATEGIES FOR APPLE A Dissertation in Horticulture by Thomas M. Kon 2016 Thomas M. Kon Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy December 2016 The dissertation of Thomas M. Kon was reviewed and approved* by the following: James R. Schupp Professor of Pomology Dissertation Advisor Chair of Committee Robert M. Crassweller Professor of Horticulture Paul Heinemann Professor and Head of Agricultural and Biological Engineering Richard Marini Professor of Horticulture Erin L. Connolly Professor of Plant Science Head of the Department of Plant Science *Signatures are on file in the Graduate School iii ABSTRACT Since 1989, approximately 150 compounds and multiple mechanical devices were evaluated as apple blossom thinners. Despite these efforts, blossom thinner adoption has been limited to a few apple producing regions or states. The purpose of this work was to: 1) compare the efficacy of promising chemical blossom thinners using a predictive model as a timing aid, and 2) evaluate the potential of short-duration thermal treatments as a blossom thinning strategy. Using a predictive model as a timing aid, the efficacy of several promising chemical blossom thinners was evaluated at Penn State’s Fruit Research and Extension Center in Biglerville, PA. Blossom thinner effects on pollen tube growth, fruit set, and yield responses were evaluated. Calcium polysulfide and ammonium thiosulfate inhibited pollen tube growth in vivo and reduced initial fruit set. Endothal was a potent thinner, but was ineffective in reducing pollen tube growth and caused excessive leaf injury. -

Systematically Integrating DNA Information Into Breeding: the MAB
Systematically integrating DNA information into breeding: The MAB Pipeline, case studies in apple and cherry Amy Iezzoni January 31, 2013 Cornell MSU Susan Brown Amy Iezzoni (PD) Kenong Xu Jim Hancock Dechun Wang Clemson Cholani Weebadde Ksenija Gasic Gregory Reighard Univ. of Arkansas John Clark WSU Texas A&M USDA-ARS Dave Byrne Cameron Peace Nahla Bassil Dorrie Main Univ. of Minnesota Gennaro Fazio Univ. of CA-Davis Kate Evans Chad Finn Karina Gallardo Jim Luby Tom Gradziel Vicki McCracken Chengyan Yue Plant Research Intl, Carlos Crisosto Nnadozie Oraguzie Netherlands Oregon State Univ. Eric van de Weg Univ. of New Hamp. Alexandra Stone Marco Bink Tom Davis Outline of Presentation The MAB Pipeline Apple skin color Cherry flesh color The MAB Pipeline “Jewels in the Genome” - discovering, polishing, applying QTL discovery MAB Pipelining Breeding (looks promising...) (polishing...) (assembling into masterpieces) Socio-Economics Surveys (example for apple) Washington Michigan Market Breeders Producers Producers Intermediaries Fruit flavor 43 41 23 Fruit crispness 15 23 10 Exterior color 26 Fruit firmness 6 7 5 Shelf life at retail 7 7 3 Sweetness/soluble solids 6 7 3 Sugar/acid balance 9 7 External appearance 13 No storage disorders 7 4 Disease resistance 2 5 Storage life 5 Other fruit quality…2 3 Size 3 Juiciness 2 Tartness Shape Phytonutrient Aroma % of respondents020406080100 Reference Germplasm McIntosh Melba LivelRasp Jolana Williams F_Spartan Spartan PRI14-126 Starr OR38T610 F_Williams NJ53 PRI14-226 Minnesota Delicious KidsOrRed -

A Manual Key for the Identification of Apples Based on the Descriptions in Bultitude (1983)
A MANUAL KEY FOR THE IDENTIFICATION OF APPLES BASED ON THE DESCRIPTIONS IN BULTITUDE (1983) Simon Clark of Northern Fruit Group and National Orchard Forum, with assistance from Quentin Cleal (NOF). This key is not definitive and is intended to enable the user to “home in” rapidly on likely varieties which should then be confirmed in one or more of the manuals that contain detailed descriptions e.g. Bunyard, Bultitude , Hogg or Sanders . The varieties in this key comprise Bultitude’s list together with some widely grown cultivars developed since Bultitude produced his book. The page numbers of Bultitude’s descriptions are included. The National Fruit Collection at Brogdale are preparing a list of “recent” varieties not included in Bultitude(1983) but which are likely to be encountered. This list should be available by late August. As soon as I receive it I will let you have copy. I will tabulate the characters of the varieties so that you can easily “slot them in to” the key. Feedback welcome, Tel: 0113 266 3235 (with answer phone), E-mail [email protected] Simon Clark, August 2005 References: Bultitude J. (1983) Apples. Macmillan Press, London Bunyard E.A. (1920) A Handbook of Hardy Fruits; Apples and Pears. John Murray, London Hogg R. (1884) The Fruit Manual. Journal of the Horticultural Office, London. Reprinted 2002 Langford Press, Wigtown. Sanders R. (1988) The English Apple. Phaidon, Oxford Each variety is categorised as belonging to one of eight broad groups. These groups are delineated using skin characteristics and usage i.e. whether cookers, (sour) or eaters (sweet). -

Participating Deltacare USA Dental Offices / Consultorios Dentales De La Red Deltacare USA - California
Participating DeltaCare USA Dental Offices / Consultorios dentales de la red DeltaCare USA - California Additional Dental Offices will be added as required. You may call our Customer Service department at 800-422-4234 for updates to the provider list. If any office is closed to further enrollment, Delta Dental reserves the right to assign you to another office as close to your home as possible. / Se agregaran mas Oficinas Dentales adicionales conforme se vayan necesitando. Puede llamar al departamento de Servicio al Cliente al 800-422-4234 para obtener una lista de los proveedores. Delta Dental se reserva el derecho de asignarle otra oficina dental lo mas cercana a su casa como sea posible. Please visit our website at deltadentalins.com for the most current listing of dentists. / Por favor visite nuestro sitio web en deltadentalins.com para la lista más actualizada de dentistas. NOTE: Contact the provider before making your choice if you have scheduling problems or small children. / NOTA: Contacte al proveedor antes de escogerlo si tiene problemas o niños pequeños. LANGUAGE ASSISTANCE: Language capabilities are self-reported by the individual dental facilities and not independently verified by Delta Dental. If an enrollee requires language assistance to enable communication in a dental setting, Delta Dental will arrange for professional services through a certified interpretation vendor at no cost to the enrollee. / ATENCIÓN EN OTROS IDIOMAS: Las habilidades en idiomas son informadas por las instalaciones dentales individuales y no son verificadas independientemente por Delta Dental. Si un afiliado requiere ayuda en otro idioma para poder comunicarse durante la cita dental, Delta Dental coordinará los servicios profesionales mediante un proveedor certificado de interpretación sin costo para el afiliado. -

Rank Dog Shows
RANKING OF DOG SHOWS, AGILITY TRIALS, OBEDIENCE TRIALS AND RALLY TRIALS SHOW AND LOCATION DOGS IN POSITION COMPETITION ALL BREED SHOWS 2006 2005 2006 2005 Anniston KC,(11/24),Montgomery,AL............................... 793 588 836 1174 Anniston KC,(04/28),Montgomery,AL............................... 622 597 1098 1160 TOP 25 ALL BREED SHOWS BY ENTRY Anoka Co MN KC,(06/23),Cambridge,MN....................... 966 916 605 694 Anoka Co MN KC,(06/22),Cambridge,MN....................... 882 842 717 784 Antelope Vly KC,(05/28),Pomona,CA.............................. 2002 2141 74 52 SHOW AND LOCATION DOGS IN POSITION COMPETITION Antelope Vly KC,(11/05),Irwindale,CA............................. 1514 1450 213 247 2006 2005 2006 2005 Apple Vly KC,(03/12),Victorville,CA ................................. 742 929 919 683 Apple Vly KC,(03/11),Victorville,CA ................................. 692 935 987 676 Arkansas KC,(06/24),Little Rock,AR................................ 769 852 876 771 Kennel Cl Palm Springs,(01/07),Indio,CA ....................... 3523 3319 1 3 Arkansas KC,(06/25),Little Rock,AR................................ 748 798 911 845 Louisville KC,(03/17),Louisville,KY.................................. 3521 3368 2 2 Asheville KC,(06/10),Fletcher,NC.................................... 1351 1288 286 329 Evansville KC,(03/18),Louisville,KY................................. 3454 3373 3 1 Asheville KC,(06/11),Fletcher,NC .................................... 1292 1204 323 374 Kennel Cl Palm Springs,(01/08),Indio,CA ....................... 3345 3080 4 6 Ashtabula KC,(07/15),Madison,OH ................................. 1047 1098 514 472 Louisville KC,(03/19),Louisville,KY.................................. 3277 3229 5 4 Ashtabula KC,(01/15),Hamburg,NY ................................ 1000 989 572 596 Harrisburg KC,(04/15),Harrisburg,PA .............................. 3104 3107 6 5 Atlanta KC,(04/15),Perry,GA........................................... -

Sfida Mondiale a Golden Delicious: Quali Alternative Possibili?
Tecnica SPECIALE MELO Sfida mondiale a Golden Delicious: quali alternative possibili? WALTER GUERRA Centro di Sperimentazione Agraria e Forestale – Laimburg (Bz) view (Belrose Inc., 2010) e raffigurati lizzati in quei tempi che inducevano Golden Delicious ha finora in tabella 2. Al Mondo, esclusa la Cina forte rugginosità sui frutti di Golden resistito alla concorrenza di che con i suoi quantitativi e la predo- Delicious. Tra le mele a buccia gialla numerose varietà di mele minanza di Fuji distorce qualunque sta- in quei tempi dominavano ancora Re- gialle. Non mancano però altre tistica, nel 2010 la varietà più prodotta netta Champagne e Renetta Ananas e, varietà simili, migliorative di era Golden Delicious, con uno “share” in misura minore, Calvilla bianca d’In- alcuni caratteri distintivi: dalla del 18,2%, equivalente ad una pro- verno, Napoleone-Koestlicher, Rosma- duzione complessiva di 4.931.000 t. rina Bianca e Belfiore Giallo. resistenza alla ticchiolatura, alla Nella graduatoria seguono parec- Dagli anni ’60, con l’introduzione sensibilità alle ammaccature, chie varietà rosse e bicolori per far ri- di nuove strategie di difesa, con l’au- dalla rugginosità della buccia comparire rispettivamente al 27° ed al mento delle conoscenze agronomiche alla fragranza della polpa. 35° posto le prossime cultivar a buc- e tecniche riguardo alla coltivazione La sfida continua. cia gialla, ovvero le giapponesi Orin della Golden Delicious e con la sco- e Mutsu. Le previsioni degli esperti perta di mutazioni meno sensibili alla americani per il 2020 piazzano Gol- rugginosità, iniziò la grande ascesa al den Delicious ancora al primo posto primato della Golden Delicious in Alto l consumatore di oggi si vede con- nella graduatoria con addirittura un Adige, in Europa e nel Mondo intero.