Spatial Scale on Recruitment and Adult Abundance In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

British Virgin Islands
British Virgin Islands Clive Petrovic, Esther Georges and Nancy Woodfield Andy McGowan Great Tobago General introduction The British Virgin Islands comprise more than 60 islands, and the Virgin Islands. These include the globally cays and rocks, with a total land area of approximately 58 threatened Cordia rupicola (CR), Maytenus cymosa (EN) and square miles (150 square km). This archipelago is located Acacia anegadensis (CR). on the Puerto Rican Bank in the north-east Caribbean at A quarter of the 24 reptiles and amphibians identified are approximately 18˚N and 64˚W. The islands once formed a endemic, including the Anegada Rock Iguana Cyclura continuous land mass with the US Virgin Islands and pinguis (CR), which is now restricted to Anegada. Other Puerto Rico, and were isolated only in relatively recent endemics include Anolis ernestwilliamsii, Eleutherodactylus geologic time. With the exception of the limestone island of schwartzi, the Anegada Ground Snake Alsophis portoricensis Anegada, the islands are volcanic in origin and are mostly anegadae, the Virgin Gorda Gecko Sphaerodactylus steep-sided with rugged topographic features and little flat parthenopian, the Virgin Gorda Worm Snake Typlops richardi land, surrounded by coral reefs. naugus, and the Anegada Worm Snake Typlops richardi Situated at the eastern end of the Greater Antilles chain, the catapontus. Other globally threatened reptiles within the islands experience a dry sub-tropical climate dominated by BVI include the Anolis roosevelti (CR) and Epicrates monensis the prevailing north-east trade winds. Maximum summer granti (EN). temperatures reach 31˚C; minimum winter temperatures Habitat alteration during the plantation era and the are 19˚C, and there is an average rainfall of 700 mm per introduction of invasive alien species has had major year with seasonal hurricane events. -

Sandy Point, Green Cay and Buck Island National Wildlife Refuges Comprehensive Conservation Plan
Sandy Point, Green Cay and Buck Island National Wildlife Refuges Comprehensive Conservation Plan U.S. Department of the Interior Fish and Wildlife Service Southeast Region September 2010 Sandy Point, Green Cay, and Buck Island National Wildlife Refuges COMPREHENSIVE CONSERVATION PLAN SANDY POINT, GREEN CAY AND BUCK ISLAND NATIONAL WILDLIFE REFUGES United States Virgin Islands Caribbean Islands National Wildlife Refuge Complex U.S. Department of the Interior Fish and Wildlife Service Southeast Region Atlanta, Georgia September 2010 Table of Contents iii Sandy Point, Green Cay, and Buck Island National Wildlife Refuges TABLE OF CONTENTS COMPREHENSIVE CONSERVATION PLAN EXECUTIVE SUMMARY ....................................................................................................................... 1 I. BACKGROUND ................................................................................................................................. 3 Introduction ................................................................................................................................... 3 Purpose and Need for the Plan .................................................................................................... 3 U.S. Fish and Wildlife Service ...................................................................................................... 3 National Wildlife Refuge System .................................................................................................. 4 Legal and Policy Context ............................................................................................................. -

ATOLL RESEARCH BULLETIN NO. 251 BIOGEOGRAPHY of the PUERTO RICAN BANK by Harold Heatwole, Richard Levins and Michael D. Byer
ATOLL RESEARCH BULLETIN NO. 251 BIOGEOGRAPHY OF THE PUERTO RICAN BANK by Harold Heatwole, Richard Levins and Michael D. Byer Issued by THE SMITHSONIAN INSTITUTION Washington, D. C., U.S.A. July 1981 VIRGIN ISLANDS CULEBRA PUERTO RlCO Fig. 1. Map of the Puerto Rican Island Shelf. Rectangles A - E indicate boundaries of maps presented in more detail in Appendix I. 1. Cayo Santiago, 2. Cayo Batata, 3. Cayo de Afuera, 4. Cayo de Tierra, 5. Cardona Key, 6. Protestant Key, 7. Green Key (st. ~roix), 8. Caiia Azul ATOLL RESEARCH BULLETIN 251 ERRATUM The following caption should be inserted for figure 7: Fig. 7. Temperature in and near a small clump of vegetation on Cayo Ahogado. Dots: 5 cm deep in soil under clump. Circles: 1 cm deep in soil under clump. Triangles: Soil surface under clump. Squares: Surface of vegetation. X's: Air at center of clump. Broken line indicates intervals of more than one hour between measurements. BIOGEOGRAPHY OF THE PUERTO RICAN BANK by Harold Heatwolel, Richard Levins2 and Michael D. Byer3 INTRODUCTION There has been a recent surge of interest in the biogeography of archipelagoes owing to a reinterpretation of classical concepts of evolution of insular populations, factors controlling numbers of species on islands, and the dynamics of inter-island dispersal. The literature on these subjects is rapidly accumulating; general reviews are presented by Mayr (1963) , and Baker and Stebbins (1965) . Carlquist (1965, 1974), Preston (1962 a, b), ~ac~rthurand Wilson (1963, 1967) , MacArthur et al. (1973) , Hamilton and Rubinoff (1963, 1967), Hamilton et al. (1963) , Crowell (19641, Johnson (1975) , Whitehead and Jones (1969), Simberloff (1969, 19701, Simberloff and Wilson (1969), Wilson and Taylor (19671, Carson (1970), Heatwole and Levins (1973) , Abbott (1974) , Johnson and Raven (1973) and Lynch and Johnson (1974), have provided major impetuses through theoretical and/ or general papers on numbers of species on islands and the dynamics of insular biogeography and evolution. -

Yachtcharter - Yachtcharter Tortola
VPM Yachtcharter - Yachtcharter Tortola Yacht - charter Yachtcharter Tortola Tortola has been a cradle of yachting for half a century now. The archipelago of the Virgin Islands seems to be created to fulfill all the wishes of those who cannot get enough of smooth sailing trips. Protected by a chain of small islands with innumerable beaches, the waters are always calm here, the trade winds blow steadily and the places to drop anchor are calm. The numerous restaurants and bars offer the sailors a comfort that is unique in the Caribbean. The beauty of the landscape, the security of the waters and the hospitality of the inhabitants make the Virgin Islands to a favorite sailing destination for yachtcharter. On a Yachtcharter starting in Tortola you will find small distances that will allow you to perform navigation on sight. The Caribbean is the ideal place for newcomers, families and those who like to enjoy. For a trip to the Virgins, which are located on US territory, you need a visa. Our VPM - Yachtcharter base in Tortola is located in Nanny Cay near Road Town. view map in fullscreen Sailing Weather Tortola: Since the islands are located in the Passat belt, the wind blows steadily from November to May from NO. In autumn and summer, however, he turns to O to SO. A constant wind sailing is therefore to be expected. In winter it can be cold fronts with stormy winds from N to NW. The hurricane season is from August to October. Best Sailing time Tortola: November to mid-April Airports near your sailing area Tortola: Tortola (EIS) - Nanny Cay: about 20 km Necessary licenses for your cruise Tortola: A special license is not required, but a sailing experience detection. -

British Virgin Islands
THE NATIONAL REPORT EL REPORTE NACIONAL FOR THE COUNTRY OF POR EL PAIS DE BRITISH VIRGIN ISLANDS NATIONAL REPRESENTATIVE / REPRESENTANTE NACIONAL LOUIS WALTERS Western Atlantic Turtle Symposium Simposio de Tortugas del Atlantico Occidental 17-22 July / Julio 1983 San José, Costa Rica BVI National Report, WATS I Vol 3, pages 70-117 WESTERN ATLANTIC TURTLE SYMPOSIUM San José, Costa Rica, July 1983 NATIONAL REPORT FOR THE COUNTRY OF BRITISH VIRGIN ISLANDS NATIONAL REPORT PRESENTED BY Louis Walters The National Representative Address: Permanent Secretary, Ministry of National Resources and Environment Tortola, British Virgin Islands NATIONAL REPORT PREPARED BY John Fletemeyer DATE SUBMITTED: 2 June 1983 Please submit this NATIONAL REPORT no later than 1 December 1982 to: IOC Assistant Secretary for IOCARIBE ℅ UNDP, Apartado 4540 San José, Costa Rica BVI National Report, WATS I Vol 3, pages 70-117 With a grant from the U.S. National Marine Fisheries Service, WIDECAST has digitized the data- bases and proceedings of the Western Atlantic Turtle Symposium (WATS) with the hope that the revitalized documents might provide a useful historical context for contemporary sea turtle management and conservation efforts in the Western Atlantic Region. With the stated objective of serving “as a starting point for the identification of critical areas where it will be necessary to concentrate all efforts in the future”, the first Western Atlantic Turtle Sym- posium convened in Costa Rica (17-22 July 1983), and the second in Puerto Rico four years later (12-16 October 1987). WATS I featured National Reports from 43 political jurisdictions; 37 pre- sented at WATS II. -

BRITISH VIRGIN ISLANDS 12 Days Day 1
YOUR CHARTER ITINERARY BRITISH VIRGIN ISLANDS 12 Days Day 1 Embarkation - Tortola and Soper’s Hole Another day of your yacht charter Itinerary starts with a short trip to Soper’s Hole Wharf & Marina on Tortola characterised by its powdery white-sand beaches and lush green mountains. Forested Sage Mountain National Park offers trails and sweeping views over neighbouring cays. One of the most picturesque and friendly marinas in the BVI, it has a little something for everyone with cafes, bars, shops, and restaurants in bright, welcoming colors. Stop by Pusser’s Landing, a famous local restaurant chain. Its terrace is a great spot to enjoy Caribbean food or try their famous, specialty Pusser's Painkiller cocktail blended with Royal Navy Rum. RYB | YOUR CHARTER ITINERARY | 2020 Day 2 Long Bay, Tortola Tortola’s most beautiful beaches are grouped around Long Bay. Smuggler’s Cove is a horseshoe of fine white sand ringed by verdant hills. Apple Bay is best for surfing and sailing in the winter season, while Carrot Bay is popular with the pelicans lounging under the banana and papaya groves. RYB | YOUR CHARTER ITINERARY | 2020 Day 3 Cane Garden Bay, Tortola Pull up a lounger and take in some rays on the beach of Cane Garden Bay. Restaurants and bars line the waterfront, including popular Quito’s and Myett’s where there’s often live music. While you're here, take a tour of the Callwood Distillery, a one-of-a-kind experience you won’t soon forget. Established in the 17th century, one of the oldest continuously-run rum distilleries in the Caribbean. -

Jost Van Dyke, British Virgin Islands
An Environmental Profile of the Island of Jost Van Dyke, British Virgin Islands including Little Jost Van Dyke, Sandy Cay, Green Cay and Sandy Spit This publication was made possible with funding support from: UK Foreign and Commonwealth Office Department for International Development Overseas Territories Environment Programme An Environmental Profile of the Island of Jost Van Dyke, British Virgin Islands including Little Jost Van Dyke, Sandy Cay, Green Cay and Sandy Spit An Initiative of the Jost Van Dykes (BVI) Preservation Society and Island Resources Foundation 2009 This publication was made possible by Use of Profile: Available from: the generous support of the Overseas Reproduction of this publication, or Jost Van Dykes (BVI) Preservation Territories Environment Programme portions of this publication, is Society (OTEP), UK Foreign and authorized for educational or non- Great Harbour Commonwealth Office, under a commercial purposes without prior Jost Van Dykes, VG 1160 contract between OTEP and the Jost permission of the Jost Van Dykes (BVI) British Virgin Islands Van Dykes (BVI) Preservation Society Preservation Society or Island Tel 284.540.0861 (JVDPS), for implementation of a Resources Foundation, provided the www.jvdps.org project identified as: source is fully acknowledged. www.jvdgreen.org BVI503: Jost Van Dyke’s Community- based Programme Advancing Citation: Island Resources Foundation Environmental Protection and Island Resources Foundation and Jost 1718 P Street Northwest, Suite T-4 Sustainable Development. Van Dykes (BVI) Preservation Society Washington, DC 20036 USA (2009). An Environmental Profile of the Tel 202.265.9712 The JVDPS contracted with Island Island of Jost Van Dyke, British Virgin Fax 202.232.0748 Resources Foundation to provide Islands, including Little Jost Van Dyke, www.irf.org technical services as a part of its Sandy Cay, Green Cay and Sandy agreement with OTEP, in particular to Spit. -

8 Day Itinerary Suggestion in the Bvis, from Beef Island to Beef Island, Tortola the Virgin Islands…
8 Day Itinerary Suggestion in the BVIs, from Beef Island to Beef Island, Tortola The Virgin Islands… Lush, tropical islands indented with sugar-white beaches and surrounded by deep turquoise seas. Tranquil, get-away-from-it-all islets and bays side by side with the glamour and sophistication of some of the world’s most exclusive resorts and yacht clubs. Is it any wonder that the Caribbean has always been, and still is, one of the most popular cruising grounds in the world? The chain of Caribbean islands sweeps southwards in an arc, creating stepping-stones from Florida to Venezuela. At the northern tip are the Virgin Islands. Comprising some 60 tiny islets and cays, the British islands offer excellent sailing and a relaxed, low-key atmosphere. With short passages between them it’s easy to hop from one island to another comfortably. Here you can moor in horseshoe bays of white sand beaches dotted with palm trees. The water beneath you is so clear that you have the feeling you are literally floating on air. Anegada Necker Island Saba Rock Great Camanoe Guana Island Little Jost Van Dyke Marina Cay Beef Virgin Gorda Sandy Jost Van Dyke Tortola Island Cay The Baths Soper’s Hole Salt Island Ginger Island Cooper Island St. Thomas Peter Island St. John Norman Island British Virgin Islands Day 1 Beef Island, Tortola OVERVIEW Welcome to a sailor's paradise! Laidback and low- key, the BVIs are flat-out gorgeous to explore. Hop aboard your yacht and discover secluded coves, impossibly beautiful SEE & DO Land in Tortola at Beef Island snorkelling spots, and ruggedly beautiful picturesque islands. -

Breeding Seabirds in the British Virgin Islands
Vol. 2: 15–20, 2006 ENDANGERED SPECIES RESEARCH Printed December 2006 Previously ESR 3: 1–6, 2006 Endang Species Res Published online May 9, 2006 Breeding seabirds in the British Virgin Islands Andrew McGowan1,*, Annette C. Broderick1, Shannon Gore2, Geoff Hilton3, Nancy K. Woodfield4, Brendan J. Godley1 1Centre for Ecology and Conservation, University of Exeter, Cornwall Campus, Tremough, Penryn TR10 9EZ, UK 2BVI Conservation and Fisheries Department, PO Box 3323, Road Town, Tortola, British Virgin Islands 3Royal Society for the Protection of Birds, The Lodge, Sandy SG19 2DL, UK 4BVI National Parks Trust, PO Box 860, Road Town, Tortola, British Virgin Islands ABSTRACT: Caribbean seabirds are subject to numerous threats, and population levels are thought to be at a fraction of historical levels. Despite being a well-known taxonomic group there is still a paucity of data for most seabird species on many of the Caribbean islands. We carried out detailed surveys of the seabird breeding populations in the British Virgin Islands (BVI) during the breeding seasons of 2004 and 2005. We surveyed 42 different islands and cays over the 2 yr with 60 and 63% of these having at least one breeding seabird species in 2004 and 2005, respectively. A total of 15 spe- cies of breeding seabird was recorded, one of which, the gull-billed tern Sterna nilotica, was pre- viously thought to have been extirpated. Two species, roseate tern Sterna dougallii and magnificent frigatebird Fregata magnificens, had globally significant colonies in the BVI and a further 8 species had a regionally significant population in the BVI. We discuss our findings within a global and regional conservation context and provide recommendations for ensuring the continued existence of BVI seabird populations. -
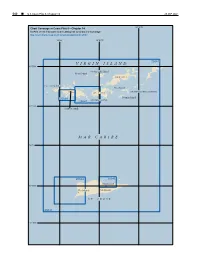
CPB5 C14 WEB.Pdf
540 ¢ U.S. Coast Pilot 5, Chapter 14 26 SEP 2021 64°30'W Chart Coverage in Coast Pilot 5—Chapter 14 NOAA’s Online Interactive Chart Catalog has complete chart coverage http://www.charts.noaa.gov/InteractiveCatalog/nrnc.shtml 65°W 64°45'W 25641 VIRGIN ISLAND 18°30'N Jost Van Dyke Island Tobago Island TORTOLA ST. THOMAS Peter Island SIR FRANCIS DRAKE CHANNEL ST. JOHN 25649 Norman Island 25647 PILLSBURY SOUND 18°15'N Charlotte Amalie MAR CARIBE 18°N 25644 25645 Christiansted 17°45'N Frederiksted Port Alucroix ST. CROIX 25641 17°30'N 26 SEP 2021 U.S. Coast Pilot 5, Chapter 14 ¢ 541 Virgin Islands (1) This chapter describes the United States Virgin peaks rising from the tableland of St. John (U.S.) to Islands, which include the islands of St. Thomas, St. heights of 800 to 1,300 feet. John and St. Croix and about 40 small islets or cays. (8) From about 20 miles north of the islands, a separation Information is given on the ports and harbors of the islands will be observed between St. Thomas and St. John, but including Charlotte Amalie, Christiansted, Port St. Croix, St. John, Jost Van Dyke, Tortola and Virgin Gorda will Cruz Bay and Frederiksted. A general description of the appear to be one large island. St. Thomas is less rugged British Virgin Islands is also included; more complete in outline than the other islands, but it may be recognized information is given in Pub. No. 147, Sailing Directions from its large midisland saddle that has horns nearly (Enroute), Caribbean Sea, Vol. -

Start - Finish Areas
Start - Finish Areas AREA APPROXIMATE RANGE/BEARING FROM NANNY CAY ENTRANCE SOL - A 2.8 NM @115° M One Design - B 0.5 NM @ 155° M Norman - C 3.2 NM @ 185° M Area - D To be Designated by Race Committee Course Marks MARK MARK NAME MARK DESCRIPTION W Windward A laid windward mark with approximate range and bearing L Leeward A laid leeward mark 1 Jost Van Dyke Jost Van Dyke, Little Jost Van Dyke, and Green Cay 2 Sandy Cay Sandy Cay and mooring buoys 3 Great Thatch Great Thatch Island 4 Little Thatch Little Thatch Island 5 Tortola Tortola, Frenchman’s Cay, Beef Island 6 Flanagan Flanagan Island 7 Pelican Pelican including the Indians and mooring bouys 8 Norman Norman Island 9 Peter Peter Island and Carrot Rock 10 Dead Chest Dead Chest and outlying rocks 11 Salt Salt Island and all mooring buoys off Lee Bay and the Rhone 12 Cooper Cooper Island and all mooring buoys off of Cooper Island Beach Club 13 Ginger Ginger Island 14 Virgin Gorda Virgin Gorda including Fallen Jerusalem and Round Rock Prickly Pear, Eustatia Island, Eustatia Sound, surrounding reef and the 15 Prickly Pear green channel marks marking Colquhoun Cut Mosquito Island including Mosquito Rock, Colquhoun Reef and the red 16 Mosquito channel marks marking Colquhoun Cut 17 Necker Necker Island 18 Seal Dogs Seal Dog and outlying rocks 19 The Dogs Great Dog, West Dog, Cockroach and George Dog islands 20 Scrub Island Scrub Island 21 Great Camanoe Great Camanoe Island 22 Guana Guana Island including mooring buoys at Monkey Point 23 FI.G.6s Gov. -

An Environmental Profile of the Island of Jost Van Dyke, British Virgin Islands
An Environmental Profile of the Island of Jost Van Dyke, British Virgin Islands Executive Summary including Little Jost Van Dyke, Sandy Cay, Green Cay and Sandy Spit This publication was made possible with funding support from: UK Foreign and Commonwealth Office Department for International Development Overseas Territories Environment Programme EXECUTIVE SUMMARY This Environmental Profile is a synthesis of the state of the environment, near the end of the first decade of the twenty-first century, in the smallest of the British Virgin Islands—the island of Jost Van Dyke*—and in the immediately adjacent smaller islands of Little Jost Van Dyke, Green Cay, Sandy Cay, and Sandy Spit. It is the first such effort in the British Virgin Islands. The Profile opens with an introductory description of the Profile islands (Chapter 1), focusing on their natural and physical features as well as the community setting, in order to provide the reader with an overview of “the place”. This is followed by a second introductory chapter (Chapter 2) which focuses on the institutional and legal framework for protecting and managing the environment in the BVI, particularly as resource management institutions and laws impact Jost Van Dyke and its satellite islands. At the end of each of the six chapters that follow (Chapters 3-8) is a section in a double-column, framed format that highlights the priority resource management and protection issues surrounding each chapter’s central theme; alongside each issue are specific recommendations for action. Chapters 3 and 4 focus on the terrestrial and marine natural resource sectors. “Conserving the Biodiversity of Jost Van Dyke” (Chapter 3) provides the first comprehensive inventory and assessment of the terrestrial environment of Jost Van Dyke, encompassing separate sections on floral and faunal resources and a further section on salt ponds as a critical ecosystem.