Materials Characterization and Analysis: Applications to Microelectronics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gas Chromatography-Mass Spectroscopy
Gas Chromatography-Mass Spectroscopy Introduction Gas chromatography-mass spectroscopy (GC-MS) is one of the so-called hyphenated analytical techniques. As the name implies, it is actually two techniques that are combined to form a single method of analyzing mixtures of chemicals. Gas chromatography separates the components of a mixture and mass spectroscopy characterizes each of the components individually. By combining the two techniques, an analytical chemist can both qualitatively and quantitatively evaluate a solution containing a number of chemicals. Gas Chromatography In general, chromatography is used to separate mixtures of chemicals into individual components. Once isolated, the components can be evaluated individually. In all chromatography, separation occurs when the sample mixture is introduced (injected) into a mobile phase. In liquid chromatography (LC), the mobile phase is a solvent. In gas chromatography (GC), the mobile phase is an inert gas such as helium. The mobile phase carries the sample mixture through what is referred to as a stationary phase. The stationary phase is usually a chemical that can selectively attract components in a sample mixture. The stationary phase is usually contained in a tube of some sort called a column. Columns can be glass or stainless steel of various dimensions. The mixture of compounds in the mobile phase interacts with the stationary phase. Each compound in the mixture interacts at a different rate. Those that interact the fastest will exit (elute from) the column first. Those that interact slowest will exit the column last. By changing characteristics of the mobile phase and the stationary phase, different mixtures of chemicals can be separated. -

Electrochemical Real-Time Mass Spectrometry: a Novel Tool for Time-Resolved Characterization of the Products of Electrochemical Reactions
Electrochemical real-time mass spectrometry: A novel tool for time-resolved characterization of the products of electrochemical reactions Elektrochemische Realzeit-Massenspektrometrie: Eine neuartige Methode zur zeitaufgelösten Charakterisierung der Produkte elektrochemischer Reaktionen Der Technischen Fakultät der Friedrich-Alexander-Universität Erlangen-Nürnberg zur Erlangung des Doktorgrades Dr.-Ingenieur vorgelegt von Peyman Khanipour Mehrin aus Shiraz, Iran Als Dissertation genehmigt von der Technischen Fakultät der Friedrich-Alexander-Universität Erlangen-Nürnberg Tag der mündlichen Prüfung: 17.11.2020 Vorsitzender des Promotionsorgans: Prof. Dr.-Ing. habil. Andreas Paul Fröba Gutachter: Prof. Dr. Karl J.J. Mayrhofer Prof. Dr. Frank-Michael Matysik I Acknowledgements This study is done in the electrosynthesis team of the electrocatalysis unit at Helmholtz- Institut Erlangen-Nürnberg (HI ERN) with the financial support of Forschungszentrum Jülich. I would like to express my deep gratitude to Prof. Dr. Karl J. J. Mayrhofer for accepting me as a Ph.D. student and also for all his encouragement, supports, and freedoms during my study. I’m grateful to Prof. Dr. Frank-Michael Matysik for kindly accepting to act as a second reviewer and also for the time he has invested in reading this thesis. This piece of work is enabled by collaboration with scientists from different expertise. I would like to express my appreciation to Dr. Sandra Haschke from FAU for providing shape-controlled high surface area platinum electrodes which I used for performing oxidation of primary alcohols and also the characterization of the provided material SEM, EDX, and XRD. Mr. Mario Löffler from HI ERN for obtaining the XPS data and his remarkable knowledge with the interpretation of the spectra on copper-based electrodes for the CO 2 electroreduction reaction. -

Ultra-Performance Liquid Chromatography Coupled to Quadrupole-Orthogonal Time-Of-flight Mass Spectrometry
RAPID COMMUNICATIONS IN MASS SPECTROMETRY Rapid Commun. Mass Spectrom. 2004; 18: 2331–2337 Published online in Wiley InterScience (www.interscience.wiley.com). DOI: 10.1002/rcm.1627 Ultra-performance liquid chromatography coupled to quadrupole-orthogonal time-of-flight mass spectrometry Robert Plumb1*, Jose Castro-Perez2, Jennifer Granger1, Iain Beattie3, Karine Joncour3 and Andrew Wright3 1Waters Corporation, Milford, MA, USA 2Waters Corporation, MS Technology Center, Manchester, UK 3AstraZeneca R&D Charnwood, Physical & Metabolic Science, Loughborough, UK Received 19 June 2004; Revised 5 August 2004; Accepted 9 August 2004 Ultra-performance liquid chromatography (UPLC) utilizes sub-2 mm particles with high linear sol- vent velocities to effect dramatic increases in resolution, sensitivity and speed of analysis. The reduction in particle size to below 2 mm requires instrumentation that can operate at pressures in the 6000–15 000 psi range. The typical peak widths generated by the UPLC system are in the order of 1–2 s for a 10-min separation. In the present work this technology has been applied to the study of in vivo drug metabolism, in particular the analysis of drug metabolites in bile. The reduction in peak width significantly increases analytical sensitivity by three- to five-fold, and the reduction in peak width, and concomitant increase in peak capacity, significantly reduces spectral overlap resulting in superior spectral quality in both MS and MS/MS modes. The application of UPLC/ MS resulted in the detection of additional drug metabolites, superior separation and improved spectral quality. Copyright # 2004 John Wiley & Sons, Ltd. The detection and identification of drug metabolites is crucial Liquid chromatography/mass spectrometry (LC/MS) and to both the drug discovery and development processes, LC/MS/MS have become the mainstay of the drug metabo- although in these two areas the emphasis is slightly different. -

Coupling Gas Chromatography to Mass Spectrometry
Coupling Gas Chromatography to Mass Spectrometry Introduction The suite of gas chromatographic detectors includes (roughly in order from most common to the least): the flame ionization detector (FID), thermal conductivity detector (TCD or hot wire detector), electron capture detector (ECD), photoionization detector (PID), flame photometric detector (FPD), thermionic detector, and a few more unusual or VERY expensive choices like the atomic emission detector (AED) and the ozone- or fluorine-induce chemiluminescence detectors. All of these except the AED produce an electrical signal that varies with the amount of analyte exiting the chromatographic column. The AED does that AND yields the emission spectrum of selected elements in the analytes as well. Another GC detector that is also very expensive but very powerful is a scaled down version of the mass spectrometer. When coupled to a GC the detection system itself is often referred to as the mass selective detector or more simply the mass detector. This powerful analytical technique belongs to the class of hyphenated analytical instrumentation (since each part had a different beginning and can exist independently) and is called gas chromatograhy/mass spectrometry (GC/MS). Placed at the end of a capillary column in a manner similar to the other GC detectors, the mass detector is more complicated than, for instance, the FID because of the mass spectrometer's complex requirements for the process of creation, separation, and detection of gas phase ions. A capillary column is required in the chromatograph because the entire MS process must be carried out at very low pressures (~10-5 torr) and in order to meet this requirement a vacuum is maintained via constant pumping using a vacuum pump. -

Fractographic Characterization of Polycarbonate Failure Modes
FRACTOGRAPHIC CHARACTERIZATION OF POLYCARBONATE FAILURE MODES Jeffrey A. Jansen, Stork Technimet Now with The Madison Group Abstract subsequently conducted in order to understand and Polycarbonate is an important plastic molding resin used to document the resulting fracture surface features. This study fabricate many engineered components. Because of its was conducted to further the understanding of the failure widespread usage, many different types of failures can mechanisms routinely observed with polycarbonate parts. result from various service conditions. Evaluating these failures through a systematic analysis program allows an Experimental assessment of how and why the parts failed. An essential A commercially available medium-viscosity polycarbonate portion of the failure analysis process is the fractographic resin was selected for the investigation. The resin was examination, which provides information about the crack molded into plaques by a custom injection molder and origin location, and the crack initiation and extension subsequently machined to form the required test specimens. modes. The focus of this investigation was to characterize The prepared fracture surfaces were examined using a the surfaces of intentionally cracked laboratory samples in Hitachi S-3500N scanning electron microscope (SEM). order to gain a more thorough understanding of The specimens were blown off and cleaned ultrasonically in polycarbonate fracture mechanisms. This paper will a mixture of isopropanol and deionized water. Prior to the document some of the key fracture features associated with inspection, the samples were gold sputter coated to enhance various polycarbonate failure modes. the imaging. Background Tests and Results Polycarbonate is a key molding resin used to produce Uniaxial Tensile Loading engineered components in numerous applications, including A uniaxial fracture was created through tensile testing using the medical, appliance, and automotive industries. -

Electron Ionization
Chapter 6 Chapter 6 Electron Ionization I. Introduction ......................................................................................................317 II. Ionization Process............................................................................................317 III. Strategy for Data Interpretation......................................................................321 1. Assumptions 2. The Ionization Process IV. Types of Fragmentation Pathways.................................................................328 1. Sigma-Bond Cleavage 2. Homolytic or Radical-Site-Driven Cleavage 3. Heterolytic or Charge-Site-Driven Cleavage 4. Rearrangements A. Hydrogen-Shift Rearrangements B. Hydride-Shift Rearrangements V. Representative Fragmentations (Spectra) of Classes of Compounds.......... 344 1. Hydrocarbons A. Saturated Hydrocarbons 1) Straight-Chain Hydrocarbons 2) Branched Hydrocarbons 3) Cyclic Hydrocarbons B. Unsaturated C. Aromatic 2. Alkyl Halides 3. Oxygen-Containing Compounds A. Aliphatic Alcohols B. Aliphatic Ethers C. Aromatic Alcohols D. Cyclic Ethers E. Ketones and Aldehydes F. Aliphatic Acids and Esters G. Aromatic Acids and Esters 4. Nitrogen-Containing Compounds A. Aliphatic Amines B. Aromatic Compounds Containing Atoms of Nitrogen C. Heterocyclic Nitrogen-Containing Compounds D. Nitro Compounds E. Concluding Remarks on the Mass Spectra of Nitrogen-Containing Compounds 5. Multiple Heteroatoms or Heteroatoms and a Double Bond 6. Trimethylsilyl Derivative 7. Determining the Location of Double Bonds VI. Library -

Fundamentals of Biological Mass Spectrometry and Proteomics
Fundamentals of Biological Mass Spectrometry and Proteomics Steve Carr Broad Institute of MIT and Harvard Modern Mass Spectrometer (MS) Systems Orbitrap Q-Exactive Triple Quadrupole Discovery/Global Experiments Targeted MS MS systems used for proteomics have 4 tasks: • Create ions from analyte molecules • Separate the ions based on charge and mass • Detect ions and determine their mass-to-charge • Select and fragment ions of interest to provide structural information (MS/MS) Electrospray MS: ease of coupling to liquid-based separation methods has made it the key technology in proteomics Possible Sample Inlets Syringe Pump Sample Injection Loop Liquid Autosampler, HPLC Capillary Electrophoresis Expansion of the Ion Formation and Sampling Regions Nitrogen Drying Gas Electrospray Atmosphere Vacuum Needle 3- 5 kV Liquid Nebulizing Gas Droplets Ions Containing Solvated Ions Isotopes Most elements have more than one stable isotope. For example, most carbon atoms have a mass of 12 Da, but in nature, 1.1% of C atoms have an extra neutron, making their mass 13 Da. Why do we care? Mass spectrometers “see” the isotope peaks provided the resolution is high enough. If an MS instrument has resolution high enough to resolve these isotopes, better mass accuracy is achieved. Stable isotopes of most abundant elements of peptides Element Mass Abundance H 1.0078 99.985% 2.0141 0.015 C 12.0000 98.89 13.0034 1.11 N 14.0031 99.64 15.0001 0.36 O 15.9949 99.76 16.9991 0.04 17.9992 0.20 Monoisotopic mass and isotopes We use instruments that resolve the isotopes enabling us to accurately measure the monoisotopic mass MonoisotopicMonoisotopic mass; all 12C, mass no 13C atoms corresponds to 13 lowestOne massC atom peak Two 13C atoms Angiotensin I (MW = 1295.6) (M+H)+ = C62 H90 N17 O14 TheWhen monoisotopic the isotopes mass of aare molecule clearly is the resolved sum of the the accurate monoisotopic masses for the massmost abundant isotope of each element present. -
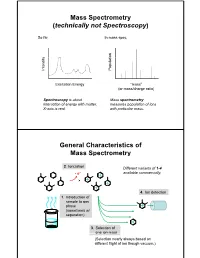
Mass Spectrometry (Technically Not Spectroscopy)
Mass Spectrometry (technically not Spectroscopy) So far, In mass spec, on y Populati Intensit Excitation Energy “mass” (or mass/charge ratio) Spectroscopy is about Mass spectrometry interaction of energy with matter. measures population of ions X-axis is real. with particular mass. General Characteristics of Mass Spectrometry 2. Ionization Different variants of 1-4 -e- available commercially. 4. Ion detection 1. Introduction of sample to gas phase (sometimes w/ separation) 3. Selection of one ion mass (Selection nearly always based on different flight of ion though vacuum.) General Components of a Mass Spectrometer Lots of choices, which can be mixed and matched. direct injection The Mass Spectrum fragment “daughter” ions M+ “parent” mass Sample Introduction: Direc t Inser tion Prob e If sample is a liquid, sample can also be injected directly into ionization region. If sample isn’t pure, get multiple parents (that can’t be distinguished from fragments). Capillary Column Introduction Continous source of molecules to spectrometer. detector column (including GC, LC, chiral, size exclusion) • Signal intensity depends on both amount of molecule and ionization efficiency • To use quantitatively, must calibrate peaks with respect eltilution time ttlitotal ion curren t to quantity eluted (TIC) over time Capillary Column Introduction Easy to interface with gas or liquid chromatography. TIC trace elution time time averaged time averaged mass spectrum mass spectrum Methods of Ionization: Electron Ionization (EI) 1 - + - 1 M + e (kV energy) M + 2e Fragmentation in Electron Ionization daughter ion (observed in spectrum) neutral fragment (not observed) excited parent at electron at electron energy of energy of 15 eV 70 e V Lower electron energy yields less fragmentation, but also less signal. -

Cryogenic Micro-Calorimeters for Mass Spectrometry of Kev Neutral Atoms
Journal of Physics: Conference Series PAPER • OPEN ACCESS Related content - The status of the micro-calorimeter at the Cryogenic micro-calorimeters for mass Shanghai EBIT - The Variability of the Recombination of spectrometry of keV neutral atoms and molecules Hydrogen Atoms on Metal Surfaces - SQUID gradiometer for ultra-low To cite this article: O Novotný et al 2015 J. Phys.: Conf. Ser. 635 032023 temperature magnetic micro-calorimeter View the article online for updates and enhancements. This content was downloaded from IP address 129.13.72.197 on 04/11/2020 at 09:19 XXIX International Conference on Photonic, Electronic, and Atomic Collisions (ICPEAC2015) IOP Publishing Journal of Physics: Conference Series 635 (2015) 032023 doi:10.1088/1742-6596/635/3/032023 Cryogenic micro-calorimeters for mass spectrometry of keV neutral atoms and molecules O Novotn´y∗;y 1, S Allgeierz, C Enssz, A Fleischmannz, L Gamerz, D Hengstlerz, S Kempfz, C Krantz∗, A Pabingerz, C Piesz, D W Saviny, D Schwalm∗;⋄, A Wolf∗ 2 ∗ Max Planck Institute for Nuclear Physics, D-69117 Heidelberg, Germany y Columbia Astrophysics Laboratory, Columbia University, New York, NY 10027, USA z Kirchhoff Institute for Physics, Heidelberg University, D-69120 Heidelberg, Germany ⋄ Faculty of Physics, Weizmann Institute of Science, Rehovot 76100, Israel Synopsis We demonstrate the capability of micro-calorimeters to detect and mass-resolve neutral atoms and molecules at ∼ keV energies, reaching single H-atom resolution. Mass spectrometric identification of fragmen- also studied in detail the MMC response function tation products is one of the most important for molecular projectiles of masses up to 56 amu. -

An Organic Chemist's Guide to Electrospray Mass Spectrometric
molecules Review An Organic Chemist’s Guide to Electrospray Mass Spectrometric Structure Elucidation Arnold Steckel 1 and Gitta Schlosser 2,* 1 Hevesy György PhD School of Chemistry, ELTE Eötvös Loránd University, Pázmány Péter sétány 1/A, 1117 Budapest, Hungary; [email protected] 2 Department of Analytical Chemistry, ELTE Eötvös Loránd University, Pázmány Péter sétány 1/A, 1117 Budapest, Hungary * Correspondence: [email protected] Received: 16 January 2019; Accepted: 8 February 2019; Published: 10 February 2019 Abstract: Tandem mass spectrometry is an important tool for structure elucidation of natural and synthetic organic products. Fragmentation of odd electron ions (OE+) generated by electron ionization (EI) was extensively studied in the last few decades, however there are only a few systematic reviews available concerning the fragmentation of even-electron ions (EE+/EE−) produced by the currently most common ionization techniques, electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI). This review summarizes the most important features of tandem mass spectra generated by collision-induced dissociation fragmentation and presents didactic examples for the unexperienced users. Keywords: tandem mass spectrometry; MS/MS fragmentation; collision-induced dissociation; CID; ESI; structure elucidation 1. Introduction Electron ionization (EI), a hard ionization technique, is the method of choice for analyses of small (<1000 Da), nonpolar, volatile compounds. As its name implies, the technique involves ionization by electrons with ~70 eV energy. This energy is high enough to yield very reproducible mass spectra with a large number of fragments. However, these spectra frequently lack the radical type molecular ions (M+) due to the high internal energy transferred to the precursors [1]. -

Electrochemistry As an Adjunct to Mass Spectrometry in Drug Development
Application Note Application Electrochemistry as an Adjunct to Mass Spectrometry in Drug Development Ian N. Acworth, David Thomas, Paul Gamache Thermo Fisher Scientific, Chelmsford, MA 1016 Key Words Drug bioactivation, Stability and degradation, Electrochemical detection, Enhanced ionization, Drug metabolites, Reactive intermediate Goal To show how on-line HPLC-electrochemical oxidation-mass spectrometry can be harnessed to accelerate several key steps in drug development. Introduction Oxidation plays a number of critical roles in biology and drug metabolism. In addition to being the major process for generating energy in a living cell, it is the major mechanism by which drugs are metabolized. Oxidation is a contributor to drug bioactivation and idiosyncratic Experimental toxicity, and is also involved in drug stability and Applications were developed using gradient analytical or degradation. Electrochemistry (EC) when used with HPLC semi-preparative HPLC with pre- or post-column has long been recognized as a sensitive and selective electrochemical oxidation followed by mass spectrometric detection technique. Indeed more than 90% of detection. Drug stability studies used flow injection pharmaceuticals in the marketplace can be oxidized and analysis with coulometric array detection. detected using this technique. However, the inherent Sample Preparation electrochemical nature of many drugs goes beyond simple Samples were dissolved in initial mobile phase solution to analysis. This commonality of oxidation makes EC a concentration of 20 -

PG Courses at the Department of Materials Science & Engineering IIT
PG Courses at the Department of Materials Science & Engineering IIT Kanpur This document contains following information 1. Details of the compulsory courses for the M.Tech. Programme 2. Details of the courses for each of the three streams a. Structure-Characterization-Property b. Metals Processing c. Functional Materials 3. Details of the courses other than the above three streams Updated on 23rd July 2013 1 1. Details of the compulsory courses for the M.Tech. Programme 2 Department of Materials Science and Engineering Indian Institute of Technology Kanpur Course Name: Structure and Characterization of Materials Credits: 3-0-0-0-4 Course No: MSE 615 Prerequisite: None Category: Compulsory course for all M.Tech. students of MSE Department, to be offered in odd semester Course Contents: Basic crystallography and crystal structures (8 Lectures hours) Lecture Hours Periodic patterns, Lattices, Motif, Unit cells, Crystal structure, Primitive and Non- 1 primitive cells Symmetry elements and point group notations 1 Crystal systems and Bravais lattices 1 Crystallographic directions and planes, Miller indices and Weiss zone law 1 Streographic projections 1 Bonding in materials and atomic packing in metals, co-ordination number concepts 1 Covalent bonding, glasses and polymers 2 Crystal defects and their significance (12 Lectures hours) Lecture Hours Point defects and their role in materials Processing, performance and failure 1 Ionically bonded structures: Pauling’s rules and some examples 2 Point defects: thermodynamics, schottkey and Frenkel