Kin28 Depletion Increases Association of TFIID Subunits Taf1 and Taf4 With
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 Supporting Information for a Microrna Network Regulates
Supporting Information for A microRNA Network Regulates Expression and Biosynthesis of CFTR and CFTR-ΔF508 Shyam Ramachandrana,b, Philip H. Karpc, Peng Jiangc, Lynda S. Ostedgaardc, Amy E. Walza, John T. Fishere, Shaf Keshavjeeh, Kim A. Lennoxi, Ashley M. Jacobii, Scott D. Rosei, Mark A. Behlkei, Michael J. Welshb,c,d,g, Yi Xingb,c,f, Paul B. McCray Jr.a,b,c Author Affiliations: Department of Pediatricsa, Interdisciplinary Program in Geneticsb, Departments of Internal Medicinec, Molecular Physiology and Biophysicsd, Anatomy and Cell Biologye, Biomedical Engineeringf, Howard Hughes Medical Instituteg, Carver College of Medicine, University of Iowa, Iowa City, IA-52242 Division of Thoracic Surgeryh, Toronto General Hospital, University Health Network, University of Toronto, Toronto, Canada-M5G 2C4 Integrated DNA Technologiesi, Coralville, IA-52241 To whom correspondence should be addressed: Email: [email protected] (M.J.W.); yi- [email protected] (Y.X.); Email: [email protected] (P.B.M.) This PDF file includes: Materials and Methods References Fig. S1. miR-138 regulates SIN3A in a dose-dependent and site-specific manner. Fig. S2. miR-138 regulates endogenous SIN3A protein expression. Fig. S3. miR-138 regulates endogenous CFTR protein expression in Calu-3 cells. Fig. S4. miR-138 regulates endogenous CFTR protein expression in primary human airway epithelia. Fig. S5. miR-138 regulates CFTR expression in HeLa cells. Fig. S6. miR-138 regulates CFTR expression in HEK293T cells. Fig. S7. HeLa cells exhibit CFTR channel activity. Fig. S8. miR-138 improves CFTR processing. Fig. S9. miR-138 improves CFTR-ΔF508 processing. Fig. S10. SIN3A inhibition yields partial rescue of Cl- transport in CF epithelia. -

TAF10 Complex Provides Evidence for Nuclear Holo&Ndash;TFIID Assembly from Preform
ARTICLE Received 13 Aug 2014 | Accepted 2 Dec 2014 | Published 14 Jan 2015 DOI: 10.1038/ncomms7011 OPEN Cytoplasmic TAF2–TAF8–TAF10 complex provides evidence for nuclear holo–TFIID assembly from preformed submodules Simon Trowitzsch1,2, Cristina Viola1,2, Elisabeth Scheer3, Sascha Conic3, Virginie Chavant4, Marjorie Fournier3, Gabor Papai5, Ima-Obong Ebong6, Christiane Schaffitzel1,2, Juan Zou7, Matthias Haffke1,2, Juri Rappsilber7,8, Carol V. Robinson6, Patrick Schultz5, Laszlo Tora3 & Imre Berger1,2,9 General transcription factor TFIID is a cornerstone of RNA polymerase II transcription initiation in eukaryotic cells. How human TFIID—a megadalton-sized multiprotein complex composed of the TATA-binding protein (TBP) and 13 TBP-associated factors (TAFs)— assembles into a functional transcription factor is poorly understood. Here we describe a heterotrimeric TFIID subcomplex consisting of the TAF2, TAF8 and TAF10 proteins, which assembles in the cytoplasm. Using native mass spectrometry, we define the interactions between the TAFs and uncover a central role for TAF8 in nucleating the complex. X-ray crystallography reveals a non-canonical arrangement of the TAF8–TAF10 histone fold domains. TAF2 binds to multiple motifs within the TAF8 C-terminal region, and these interactions dictate TAF2 incorporation into a core–TFIID complex that exists in the nucleus. Our results provide evidence for a stepwise assembly pathway of nuclear holo–TFIID, regulated by nuclear import of preformed cytoplasmic submodules. 1 European Molecular Biology Laboratory, Grenoble Outstation, 6 rue Jules Horowitz, 38042 Grenoble, France. 2 Unit for Virus Host-Cell Interactions, University Grenoble Alpes-EMBL-CNRS, 6 rue Jules Horowitz, 38042 Grenoble, France. 3 Cellular Signaling and Nuclear Dynamics Program, Institut de Ge´ne´tique et de Biologie Mole´culaire et Cellulaire, UMR 7104, INSERM U964, 1 rue Laurent Fries, 67404 Illkirch, France. -

Structure and Mechanism of the RNA Polymerase II Transcription Machinery
Downloaded from genesdev.cshlp.org on October 9, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW Structure and mechanism of the RNA polymerase II transcription machinery Allison C. Schier and Dylan J. Taatjes Department of Biochemistry, University of Colorado, Boulder, Colorado 80303, USA RNA polymerase II (Pol II) transcribes all protein-coding ingly high resolution, which has rapidly advanced under- genes and many noncoding RNAs in eukaryotic genomes. standing of the molecular basis of Pol II transcription. Although Pol II is a complex, 12-subunit enzyme, it lacks Structural biology continues to transform our under- the ability to initiate transcription and cannot consistent- standing of complex biological processes because it allows ly transcribe through long DNA sequences. To execute visualization of proteins and protein complexes at or near these essential functions, an array of proteins and protein atomic-level resolution. Combined with mutagenesis and complexes interact with Pol II to regulate its activity. In functional assays, structural data can at once establish this review, we detail the structure and mechanism of how enzymes function, justify genetic links to human dis- over a dozen factors that govern Pol II initiation (e.g., ease, and drive drug discovery. In the past few decades, TFIID, TFIIH, and Mediator), pausing, and elongation workhorse techniques such as NMR and X-ray crystallog- (e.g., DSIF, NELF, PAF, and P-TEFb). The structural basis raphy have been complemented by cryoEM, cross-linking for Pol II transcription regulation has advanced rapidly mass spectrometry (CXMS), and other methods. Recent in the past decade, largely due to technological innova- improvements in data collection and imaging technolo- tions in cryoelectron microscopy. -

Supplementary Tables Supplementary Table S1. Treatment Protocols
Supplementary Tables Supplementary Table S1. Treatment protocols pediatric AML patients. Number of Treatment patients ADE-based regimen 64 ADE with bortezomib 14 ADE with mylotarg 6 APL-like treatment 2 TMD-like treatment 1 Relapse therapy 2 Misc treatment/novel agents 5 Unknown 2 Supplemental Table S2: Patient characteristics for the 73 pediatric acute lymphoblastic leukemia patients Characteristics N, % Number of cases 73 ALL subtype Pre-B ALL 57 (78) T-ALL 16 (22) Age, y, median (range) 7.3 (0.2-18.0) Gender Male 40 (55) Female 33 (45) Declared Ethnicity Caucasian 61 (84) Hispanic 44 (72) Non-Hispanic 17 (28) Black American 6 (8) Asian 4 (5) Mixed 2 (3) Hispanic 1 (1) ‘‘SNP’’ Ethnicity European 9 (12) African 6 (8) American Indian 38 (52) Asian 1 (1) Not done 19 (26) Cytogenetics Favorable 15 (21) Intermediate 42 (58) Unfavorable 15 (21) Unknown 1 (1) Risk Group Low Risk 4 (5) Standard/ Intermediate Risk 29 (40) High/Very High Risk 40 (55) CNS status CNS-1 46 (63) CNS-2 20 (27) CNS-3 6 (8) Unknown 2 (3) Response Complete remission 67 (92) Resistant 4 (5) Fail 2 (3) Alive 63 (86) Supplementary Table S3. The table shows the ‘‘Rosetta Stone’’ of the antibody and protein nomenclature, and the R2 for the antibody validation and the primary and secondary antibody dilutions. Secondary, all antibodies that were used in combination with information about the manufacturer of each antibody, the antibody source, and catalog number are listed. Functional Protein Name Rosetta Stone RPPA Staining Details Category Functional Effect Common Name RPPA Antibody Huge Name (added MiMI Antibody R2, WB vs. -
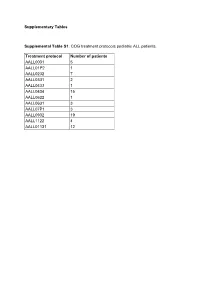
Supplementary Tables Supplemental Table S1. COG Treatment Protocols
Supplementary Tables Supplemental Table S1. COG treatment protocols pediatric ALL patients. Treatment protocol Number of patients AALL0031 5 AALL01P2 1 AALL0232 7 AALL0331 2 AALL0433 1 AALL0434 15 AALL0622 1 AALL0631 3 AALL07P1 3 AALL0932 19 AALL1122 4 AALL01131 12 Supplementary Table S2. This table contains the “Rosetta Stone” for the antibody and protein nomenclature (HUGO, MiMI, GeneCards) together with the RPPA staining details, including the primary and secondary antibody dilutions, the R2 scores For antibody validation, catalog number and the antibody source. Functional Protein Name Rosetta Stone RPPA Staining Details Category Functional Effect Common Name RPPA Antibody Huge Name (added MiMI Antibody R2, WB vs. Antibody 2nd Ab Full Name/Description from GeneCards of Mfg Claimed Manufacturer Catalog# Functional Group Name with PTMs) Name Source RPPA Dilution dilution Phosphorylation Target AKT1 AKT1 AKT1 v-akt murine thymoma viral oncogene homolog 1 AKT1 Cell Signaling 9272 Rabbit > 0.7 200 15000 PI3KAKT AKT1/AKT2/AKT3 AKT1_2_3.pS473 AKT1 v-akt murine thymoma viral oncogene homolog 1 Activation AKT-P473(Ser) Cell Signaling 9271 Rabbit > 0.7 150 20000 PI3KAKT Phospho Ser473 AKT1/AKT2/AKT3 AKT1_2_3.pT308 AKT1 v-akt murine thymoma viral oncogene homolog 1 Activation AKT-P308(Thr) Cell Signaling 9275 Rabbit > 0.7 200 20000 PI3KAKT Phospho Thr308 ASNS ASNS ASNS asparagine synthetase (glutamine-hydrolyzing) ASNS Sigma HPA029318 Rabbit 0.5-0.7 800 20000 Metabolic ATF3 ATF3 ATF3 activating transcription factor 3 ATF3 Abcam ab87213 Rabbit -

TAF4 Takes Flight
COMMENTARY TAF4 takes flight Michael T. Marr II1 Department of Biology and Rosenstiel Basic Medical Sciences Research Center, Brandeis University, Waltham, MA 02454 he eukaryotic mRNA transcrip- from yeast to man, but the region of ETO-TAFH domain was required for tion machinery is exceedingly conservation is limited to a histone H2A activation of the wingless target gene complex, perhaps reflecting the homology region at the carboxyl termi- naked cuticle (nkd) both in cultured requirement for response to nus of the protein (Fig. 1A). The meta- cells and in larval imaginal discs. They Tdiverse environmental and developmen- zoan homologues of TAF4 contain an further showed that this domain directly tal signals. Some of the machinery is extended amino terminus with two addi- interacts with the amino terminus of the common to all mRNA genes and in- tional conserved regions: a glutamine- pygopus, a dedicated transcription factor cludes RNA polymerase II (Pol II) and rich region and a more recently defined for wingless signaling. Interestingly, the a set of general transcription factors ETO-TAFH domain. amino terminus of pygopus has previ- (GTFs) (1). Signal-specific gene expres- The glutamine-rich region was one of ously been defined as absolutely neces- sion patterns are defined by sequence- the first coactivator domains of TFIID sary for Wg/Wnt signaling (14). These specific DNA-binding proteins (activa- to be defined (8). It interacts with a findings place TAF4 at the end of a tors) that bind cognate sites in the number of activators including Sp1 and chain of protein–protein interactions promoter and enhancers of target genes. -

NRF1) Coordinates Changes in the Transcriptional and Chromatin Landscape Affecting Development and Progression of Invasive Breast Cancer
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 11-7-2018 Decipher Mechanisms by which Nuclear Respiratory Factor One (NRF1) Coordinates Changes in the Transcriptional and Chromatin Landscape Affecting Development and Progression of Invasive Breast Cancer Jairo Ramos [email protected] Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Clinical Epidemiology Commons Recommended Citation Ramos, Jairo, "Decipher Mechanisms by which Nuclear Respiratory Factor One (NRF1) Coordinates Changes in the Transcriptional and Chromatin Landscape Affecting Development and Progression of Invasive Breast Cancer" (2018). FIU Electronic Theses and Dissertations. 3872. https://digitalcommons.fiu.edu/etd/3872 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida DECIPHER MECHANISMS BY WHICH NUCLEAR RESPIRATORY FACTOR ONE (NRF1) COORDINATES CHANGES IN THE TRANSCRIPTIONAL AND CHROMATIN LANDSCAPE AFFECTING DEVELOPMENT AND PROGRESSION OF INVASIVE BREAST CANCER A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in PUBLIC HEALTH by Jairo Ramos 2018 To: Dean Tomás R. Guilarte Robert Stempel College of Public Health and Social Work This dissertation, Written by Jairo Ramos, and entitled Decipher Mechanisms by Which Nuclear Respiratory Factor One (NRF1) Coordinates Changes in the Transcriptional and Chromatin Landscape Affecting Development and Progression of Invasive Breast Cancer, having been approved in respect to style and intellectual content, is referred to you for judgment. -

Short- and Long-Term Impact of Hyperoxia on the Blood and Retinal Cells’ Transcriptome in a Mouse Model of Oxygen- Induced Retinopathy
www.nature.com/pr BASIC SCIENCE ARTICLE OPEN Short- and long-term impact of hyperoxia on the blood and retinal cells’ transcriptome in a mouse model of oxygen- induced retinopathy Magdalena Zasada1, Anna Madetko-Talowska2, Cecilie Revhaug3,4, Anne Gro W. Rognlien3,4, Lars O. Baumbusch3,Teofila Książek2, Katarzyna Szewczyk2, Agnieszka Grabowska2, Miroslaw Bik-Multanowski2, Jacek Józef Pietrzyk1, Przemko Kwinta1 and Ola Didrik Saugstad3,4 BACKGROUND: We aimed to identify global blood and retinal gene expression patterns in murine oxygen-induced retinopathy (OIR), a common model of retinopathy of prematurity, which may allow better understanding of the pathogenesis of this severe ocular prematurity complication and identification of potential blood biomarkers. METHODS: A total of 120 C57BL/6J mice were randomly divided into an OIR group, in which 7-day-old pups were maintained in 75% oxygen for 5 days, or a control group. RNA was extracted from the whole-blood mononuclear cells and retinal cells on days 12, 17, and 28. Gene expression in the RNA samples was evaluated with mouse gene expression microarrays. RESULTS: There were 38, 1370 and 111 genes, the expression of which differed between the OIR and control retinas on days 12, 17, and 28, respectively. Gene expression in the blood mononuclear cells was significantly altered only on day 17. Deptor and Nol4 genes showed reduced expression both in the blood and retinal cells on day 17. 1234567890();,: CONCLUSION: There are sustained marked changes in the global pattern of gene expression in the OIR mice retinas. An altered expression of Deptor and Nol4 genes in the blood mononuclear cells requires further investigation as they may indicate retinal neovascularization. -

The Relationship Between Long-Range Chromatin Occupancy and Polymerization of the Drosophila ETS Family Transcriptional Repressor Yan
INVESTIGATION The Relationship Between Long-Range Chromatin Occupancy and Polymerization of the Drosophila ETS Family Transcriptional Repressor Yan Jemma L. Webber,*,1 Jie Zhang,*,†,1 Lauren Cote,* Pavithra Vivekanand,*,2 Xiaochun Ni,‡,§ Jie Zhou,§ Nicolas Nègre,**,3 Richard W. Carthew,†† Kevin P. White,‡,§,** and Ilaria Rebay*,†,4 *Ben May Department for Cancer Research, †Committee on Cancer Biology, ‡Department of Ecology and Evolution, §Department of Human Genetics, and **Institute for Genomics and Systems Biology, University of Chicago, Chicago, Illinois 60637, and ††Department of Molecular Biosciences, Northwestern University, Evanston, Illinois 60208 ABSTRACT ETS family transcription factors are evolutionarily conserved downstream effectors of Ras/MAPK signaling with critical roles in development and cancer. In Drosophila, the ETS repressor Yan regulates cell proliferation and differentiation in a variety of tissues; however, the mechanisms of Yan-mediated repression are not well understood and only a few direct target genes have been identified. Yan, like its human ortholog TEL1, self-associates through an N-terminal sterile a-motif (SAM), leading to speculation that Yan/TEL1 polymers may spread along chromatin to form large repressive domains. To test this hypothesis, we created a monomeric form of Yan by recombineering a point mutation that blocks SAM-mediated self-association into the yan genomic locus and compared its genome-wide chromatin occupancy profile to that of endogenous wild-type Yan. Consistent with the spreading model predictions, wild-type Yan-bound regions span multiple kilobases. Extended occupancy patterns appear most prominent at genes encoding crucial developmental regulators and signaling molecules and are highly conserved between Drosophila melanogaster and D. virilis, suggest- ing functional relevance. -

Chromatin Occupancy and Target Genes of the Haematopoietic Master Transcription Factor MYB Roza B
www.nature.com/scientificreports OPEN Chromatin occupancy and target genes of the haematopoietic master transcription factor MYB Roza B. Lemma1,2,8, Marit Ledsaak1,3,8, Bettina M. Fuglerud1,4,5, Geir Kjetil Sandve6, Ragnhild Eskeland1,3,7 & Odd S. Gabrielsen1* The transcription factor MYB is a master regulator in haematopoietic progenitor cells and a pioneer factor afecting diferentiation and proliferation of these cells. Leukaemic transformation may be promoted by high MYB levels. Despite much accumulated molecular knowledge of MYB, we still lack a comprehensive understanding of its target genes and its chromatin action. In the present work, we performed a ChIP-seq analysis of MYB in K562 cells accompanied by detailed bioinformatics analyses. We found that MYB occupies both promoters and enhancers. Five clusters (C1–C5) were found when we classifed MYB peaks according to epigenetic profles. C1 was enriched for promoters and C2 dominated by enhancers. C2-linked genes were connected to hematopoietic specifc functions and had GATA factor motifs as second in frequency. C1 had in addition to MYB-motifs a signifcant frequency of ETS-related motifs. Combining ChIP-seq data with RNA-seq data allowed us to identify direct MYB target genes. We also compared ChIP-seq data with digital genomic footprinting. MYB is occupying nearly a third of the super-enhancers in K562. Finally, we concluded that MYB cooperates with a subset of the other highly expressed TFs in this cell line, as expected for a master regulator. Te transcription factor c-Myb (approved human symbol MYB), encoded by the MYB proto-oncogene, is highly expressed in haematopoietic progenitor cells and plays a key role in regulating the expression of genes involved in diferentiation and proliferation of myeloid and lymphoid progenitors 1–5. -

CREB-Dependent Transcription in Astrocytes: Signalling Pathways, Gene Profiles and Neuroprotective Role in Brain Injury
CREB-dependent transcription in astrocytes: signalling pathways, gene profiles and neuroprotective role in brain injury. Tesis doctoral Luis Pardo Fernández Bellaterra, Septiembre 2015 Instituto de Neurociencias Departamento de Bioquímica i Biologia Molecular Unidad de Bioquímica y Biologia Molecular Facultad de Medicina CREB-dependent transcription in astrocytes: signalling pathways, gene profiles and neuroprotective role in brain injury. Memoria del trabajo experimental para optar al grado de doctor, correspondiente al Programa de Doctorado en Neurociencias del Instituto de Neurociencias de la Universidad Autónoma de Barcelona, llevado a cabo por Luis Pardo Fernández bajo la dirección de la Dra. Elena Galea Rodríguez de Velasco y la Dra. Roser Masgrau Juanola, en el Instituto de Neurociencias de la Universidad Autónoma de Barcelona. Doctorando Directoras de tesis Luis Pardo Fernández Dra. Elena Galea Dra. Roser Masgrau In memoriam María Dolores Álvarez Durán Abuela, eres la culpable de que haya decidido recorrer el camino de la ciencia. Que estas líneas ayuden a conservar tu recuerdo. A mis padres y hermanos, A Meri INDEX I Summary 1 II Introduction 3 1 Astrocytes: physiology and pathology 5 1.1 Anatomical organization 6 1.2 Origins and heterogeneity 6 1.3 Astrocyte functions 8 1.3.1 Developmental functions 8 1.3.2 Neurovascular functions 9 1.3.3 Metabolic support 11 1.3.4 Homeostatic functions 13 1.3.5 Antioxidant functions 15 1.3.6 Signalling functions 15 1.4 Astrocytes in brain pathology 20 1.5 Reactive astrogliosis 22 2 The transcription -

Molecular Targeting and Enhancing Anticancer Efficacy of Oncolytic HSV-1 to Midkine Expressing Tumors
University of Cincinnati Date: 12/20/2010 I, Arturo R Maldonado , hereby submit this original work as part of the requirements for the degree of Doctor of Philosophy in Developmental Biology. It is entitled: Molecular Targeting and Enhancing Anticancer Efficacy of Oncolytic HSV-1 to Midkine Expressing Tumors Student's name: Arturo R Maldonado This work and its defense approved by: Committee chair: Jeffrey Whitsett Committee member: Timothy Crombleholme, MD Committee member: Dan Wiginton, PhD Committee member: Rhonda Cardin, PhD Committee member: Tim Cripe 1297 Last Printed:1/11/2011 Document Of Defense Form Molecular Targeting and Enhancing Anticancer Efficacy of Oncolytic HSV-1 to Midkine Expressing Tumors A dissertation submitted to the Graduate School of the University of Cincinnati College of Medicine in partial fulfillment of the requirements for the degree of DOCTORATE OF PHILOSOPHY (PH.D.) in the Division of Molecular & Developmental Biology 2010 By Arturo Rafael Maldonado B.A., University of Miami, Coral Gables, Florida June 1993 M.D., New Jersey Medical School, Newark, New Jersey June 1999 Committee Chair: Jeffrey A. Whitsett, M.D. Advisor: Timothy M. Crombleholme, M.D. Timothy P. Cripe, M.D. Ph.D. Dan Wiginton, Ph.D. Rhonda D. Cardin, Ph.D. ABSTRACT Since 1999, cancer has surpassed heart disease as the number one cause of death in the US for people under the age of 85. Malignant Peripheral Nerve Sheath Tumor (MPNST), a common malignancy in patients with Neurofibromatosis, and colorectal cancer are midkine- producing tumors with high mortality rates. In vitro and preclinical xenograft models of MPNST were utilized in this dissertation to study the role of midkine (MDK), a tumor-specific gene over- expressed in these tumors and to test the efficacy of a MDK-transcriptionally targeted oncolytic HSV-1 (oHSV).