Acetaminophen + Ascorbic Acid + Pheniramine + Phenylephrine Acetaminophen + Chlorpheniramine + Phenylephrine Acetylaminonitropro
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tonsillopharyngitis - Acute (1 of 10)
Tonsillopharyngitis - Acute (1 of 10) 1 Patient presents w/ sore throat 2 EVALUATION Yes EXPERT Are there signs of REFERRAL complication? No 3 4 EVALUATION Is Group A Beta-hemolytic Yes DIAGNOSIS Streptococcus (GABHS) • Rapid antigen detection test infection suspected? (RADT) • roat culture No TREATMENT EVALUATION No A Supportive management Is GABHS confi rmed? B Pharmacological therapy (Non-GABHS) Yes 5 TREATMENT A EVALUATE RESPONSEMIMS Supportive management TO THERAPY C Pharmacological therapy • Antibiotics Poor/No Good D Surgery, if recurrent or complicated response response REASSESS PATIENT COMPLETE THERAPY & REVIEW THE DIAGNOSIS© Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B269 © MIMS Pediatrics 2020 Tonsillopharyngitis - Acute (2 of 10) 1 ACUTE TONSILLOPHARYNGITIS • Infl ammation of the tonsils & pharynx • Etiologies include bacterial (group A β-hemolytic streptococcus, Haemophilus infl uenzae, Fusobacterium sp, etc) & viral (infl uenza, adenovirus, coronavirus, rhinovirus, etc) pathogens • Sore throat is the most common presenting symptom in older children TONSILLOPHARYNGITIS 2 EVALUATION FOR COMPLICATIONS • Patients w/ sore throat may have deep neck infections including epiglottitis, peritonsillar or retropharyngeal abscess • Examine for signs of upper airway obstruction Signs & Symptoms of Sore roat w/ Complications • Trismus • Inability to swallow liquids • Increased salivation or drooling • Peritonsillar edema • Deviation of uvula -

List of Union Reference Dates A
Active substance name (INN) EU DLP BfArM / BAH DLP yearly PSUR 6-month-PSUR yearly PSUR bis DLP (List of Union PSUR Submission Reference Dates and Frequency (List of Union Frequency of Reference Dates and submission of Periodic Frequency of submission of Safety Update Reports, Periodic Safety Update 30 Nov. 2012) Reports, 30 Nov. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Nexobrid 2 G Powder and Gel for Gel
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions. 1. NAME OF THE MEDICINAL PRODUCT NexoBrid 2 g powder and gel for gel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 2 g of concentrate of proteolytic enzymes enriched in bromelain, corresponding to 0.09 g/g concentrate of proteolytic enzymes enriched in bromelain after mixing (or 2 g/22 g gel). The proteolytic enzymes are a mixture of enzymes from the stem of Ananas comosus (pineapple plant). For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Powder and gel for gel. The powder is off-white to light tan. The gel is clear and colourless. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications NexoBrid is indicated for removal of eschar in adults with deep partial- and full-thickness thermal burns. 4.2 Posology and method of administration NexoBrid should only be applied by trained healthcare professionals in specialist burn centres. Posology 2 g NexoBrid powder in 20 g gel is applied to a burn wound area of 100 cm2. NexoBrid should not be applied to more than 15% Total Body Surface Area (TBSA) (see also section 4.4, Coagulopathy). NexoBrid should be left in contact with the burn for a duration of 4 hours. There is very limited information on the use of NexoBrid on areas where eschar remained after the first application. -

Chewable Lozenge Formulation
Umashankar M S et al. Int. Res. J. Pharm. 2016, 7 (4) INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 – 8407 Review Article CHEWABLE LOZENGE FORMULATION- A REVIEW Umashankar M S *, Dinesh S R, Rini R, Lakshmi K S, Damodharan N SRM College of Pharmacy, SRM University, Kattankulathur, India *Corresponding Author Email: [email protected] Article Received on: 11/02/16 Revised on: 13/03/16 Approved for publication: 28/03/16 DOI: 10.7897/2230-8407.07432 ABSTRACT Development of lozenges dated back to 20thcentury and is still remain popular among the consumer and hence it has continued commercial production. Lozenges are palatable solid unit dosage form administrated in the oral cavity. They meant to be dissolved in mouth or pharynx for its local or systemic effect. Lozenge tablets provide several advantages as pharmaceutical formulations however with some disadvantages. Lozenge as a dosage form can be adopted for drug delivery across buccal route, labial route, gingival route and sublingual route. Multiple drugs can also be incorporated in them for chronic illness treatments. Lozenge enables loading of wide range of active ingredients for oral systemic delivery of drugs. Lozenges are available as over the counter medications in the form of caramel based soft lozenges, hard candy lozenges and compressed tablet lozenges containing drugs for sore throat, mouth infection and as mouth fresheners. The rationale behind the use of medicated lozenges as one of the most favored dosage form for the delivery of antitussive drugs. This review focuses various aspects of lozenge formulation providing an insight to the formulation scientist on novel application of lozenge drug delivery system. -

Substantial Equivalence Determination Decision Summary Assay Only Template
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k091024 B. Purpose for Submission: Premarket notification C. Measurand: Methicillin Resistant Staphylococcus aureus (MRSA) D. Type of Test: Detection of MRSA using a selective and differential chromogenic media E. Applicant: bioMérieux, Inc. F. Proprietary and Established Names: chromID™ MRSA Agar G. Regulatory Information: 1. Regulation section: 21 CFR 866.1700 2. Classification: Class II 3. Product code: JSO Culture media, Antimicrobial susceptibility test, excluding Mueller Hinton Agar 1 4. Panel: Microbiology H. Intended Use: 1. Intended use(s): chromID™ MRSA agar is a selective and differential chromogenic medium for the qualitative detection of nasal colonization of methicillin resistant Staphylococcus aureus (MRSA) to aid in the prevention and control of MRSA infections in healthcare settings. The test is performed on anterior nares swab specimens from patients and healthcare workers to screen for MRSA colonization. chromID™ MRSA agar is not intended to diagnose MRSA infection nor to guide or monitor treatment of infection. 2. Indication(s) for use: The chromID™ MRSA agar is not intended to diagnose MRSA infection nor to guide or monitor treatment of infection. 3. Special conditions for use statement(s): Prescription use 4. Special instrument requirements: Not applicable I. Device Description: The chromID™ MRSA agar is translucent and a light tan color. After the plates are inoculated and incubated, colonies growing on the plates will have either a green appearance, which indicates a positive MRSA status or a colorless appearance which indicates a negative MRSA status. The green color is more vivid if the colonies are observed through the agar from the bottom of the plate. -
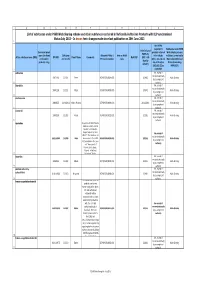
List of Substances Under PSUR WS Scheme and Other Substances
ABCDEFGHIJKL List of substances under PSUR Work Sharing scheme and other substances contained in Nationally Authorised Products with DLP synchronised Status July 2013 In brown font: changes made since last publication on 30th June 2013 1 Are PSURs required for Substances under PSUR Submission of Innovator brand products referred Work Sharing scheme PSURs by name (for fixed DLP (year Allocated PRMS / Note on PSUR to in Articles or Others (contained in Active substance name (INN) EU HBD Firm's Name Comments Next DLP (DLP + 90 combination and month) Procedure number cycle 10(1), 10a, 14 and Nationally Authorised days by products only) 16a of Directive Products including default) 2001/83/EC as MRP/DCP) 2 amended? sulbactam No, except if required nationally 19871116 201311 Pfizer AT/H/PSUR/0004/003 201402 Work Sharing by a competent 3 authority idarubicin No, except if required nationally 19891129 201311 Pfizer AT/H/PSUR/0005/003 201402 Work Sharing by a competent 4 authority dexibuprofen No, except if required nationally 20000725 31/08/2014 Gebro Pharma AT/H/PSUR/0009/003 29/11/2014 Work Sharing by a competent 5 authority nicorandil No, except if required nationally 19900209 201302 Merck AT/H/PSUR/0023/002 201305 Work Sharing by a competent 6 authority azelastine Request from MEDA Pharma GmbH & Co.KG to amend the DLP to 31/12/2014. Request agreed by the P- No, except if RMS AT. The substance has required nationally 20/11/1990 201309 Meda been moved to the EURD AT/H/PSUR/0038/001 201312 Work Sharing by a competent list as detailed in the -

Adverse Effects and Precautions
1736 Disinfectants and Preservatives i Amylmetacresoi (BAN, riNN) ..................Preparat ons........ Adverse Effects and Precautions ProprietaryPreparations (details are given in Volume B) The alkyl gallates may cause contact sensitivity and skin Aml!metacreso!; A[ililrnetakrezol: Amylrnet.acreso[; Amylrne. AM<InM<;· Multi-ingredient Preparations. Singapore: Esemdent. reactions. ta�resolurn; Amylmetakresol: Amyylirnetakresoli; TaKpe30J1. Effects on the blood. Methaemoglobinaemia associated 6-Pentyl-rn,cresol; S;Methyl.-2-pentylpheooi. Alkyl Gallates with the antoxidants (butylated hydroxyanisole, butylated C1 J1,80= 178.3 hydroxytoluene, and propyl gallate) used to preserve the CAS 1300,94-3. .de Gaiatos alquilo; AnKwnrannalJ:>t. oil in a soybean infant feed fom1ula has been reported.1 UN!! -'-· 05W904P57F: Propyl gallate was suspected of being the most likely cause Dodecyl Gallate because its chemical structure is similar to pyrogallol Pharmacopoeias. In Bur. (see p. vii). (p. 1718.2), a methaemoglobinaemia inducer. Ph. Eur. 8: (Amylmetacresol). A clear or almost clear liquid Dodecilo galatas; Dociecyle,. gallate Dodecylgallat; . de; ..•. I. Nitzan M, et al. Infantile methemoglobinemia caused by food additives. or a solid crystalline mass, colourless or slightly yellow Dodecyi-9Jilat; D(0ec%lis (iallas:. Dodekyyligallaattl; E312; Clin Toxicol 1979; 15: 273-80. when freshly prepared; it darkens or discolours to dark m Galata • de dodecflo; Laury! Gailate; Laurylum Gafikw ; yellow, brownish-yellow, or pink on keeping. F.p. about 22 }logel\vfn rarinar. Preparations degrees. Practically insoluble in water; very soluble in Dodecyi 3,4,5-trihyc;lroxybenzoate. ...................... alcohol and in acetone. Store in non�metallic airtight (details are given in Volume B) C;ul;lje0$=338.4 ProprietaryPreparations containers. Protect from light. -

Clenbuterol Elisa Kit Instructions Product #101219 & 101216 Forensic Use Only
Neogen Corporation 944 Nandino Blvd., Lexington KY 40511 USA 800/477-8201 USA/Canada | 859/254-1221 Fax: 859/255-5532 | E-mail: [email protected] | Web: www.neogen.com/Toxicology CLENBUTEROL ELISA KIT INSTRUCTIONS PRODUCT #101219 & 101216 FORENSIC USE ONLY INTENDED USE: For the determination of trace quantities of Clenbuterol and/or other metabolites in human urine, blood, oral fluid. DESCRIPTION Neogen Corporation’s Clenbuterol ELISA (Enzyme-Linked ImmunoSorbent Assay) test kit is a qualitative one-step kit designed for use as a screening device for the detection of drugs and/or their metabolites. The kit was designed for screening purposes and is intended for forensic use only. It is recommended that all suspect samples be confirmed by a quantitative method such as gas chromatography/mass spectrometry (GC/MS). ASSAY PRINCIPLES Neogen Corporation’s test kit operates on the basis of competition between the drug or its metabolite in the sample and the drug- enzyme conjugate for a limited number of antibody binding sites. First, the sample or control is added to the microplate. Next, the diluted drug-enzyme conjugate is added and the mixture is incubated at room temperature. During this incubation, the drug in the sample or the drug-enzyme conjugate binds to antibody immobilized in the microplate wells. After incubation, the plate is washed 3 times to remove any unbound sample or drug-enzyme conjugate. The presence of bound drug-enzyme conjugate is recognized by the addition of K-Blue® Substrate (TMB). After a 30 minute substrate incubation, the reaction is halted with the addition of Red Stop Solution. -

Development of Granulation Tissue Mimetic Scaffolds for Skin Healing
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 10-4-2018 12:30 PM Development Of Granulation Tissue Mimetic Scaffolds For Skin Healing Adam Hopfgartner The University of Western Ontario Supervisor Hamilton, Douglas W. The University of Western Ontario Co-Supervisor Pickering, J. Geoffrey. The University of Western Ontario Graduate Program in Biomedical Engineering A thesis submitted in partial fulfillment of the equirr ements for the degree in Master of Engineering Science © Adam Hopfgartner 2018 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Biomaterials Commons, and the Molecular, Cellular, and Tissue Engineering Commons Recommended Citation Hopfgartner, Adam, "Development Of Granulation Tissue Mimetic Scaffolds For Skin Healing" (2018). Electronic Thesis and Dissertation Repository. 5767. https://ir.lib.uwo.ca/etd/5767 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Abstract Impaired skin healing is a significant and growing clinical concern, particularly in relation to diabetes, venous insufficiency and immobility. Previously, we developed electrospun scaffolds for the delivery of periostin (POSTN) and connective tissue growth factor 2 (CCN2), matricellular proteins involved in the proliferative phase of healing. This study aimed to design and validate a novel electrosprayed coaxial microsphere for the encapsulation of fibroblast growth factor 9 (FGF9), as a component of the POSTN/CCN2 scaffold, to promote angiogenic stability during wound healing. For the first time, we observed a pro-proliferative effect of FGF9 on human dermal fibroblasts (HDF) in vitro, indicating a potential cellular mechanism of action during wound healing. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Supplementary Information
1 SUPPLEMENTARY INFORMATION 2 ATIQ – further information 3 The Asthma Treatment Intrusiveness Questionnaire (ATIQ) scale was adapted from a scale originally 4 developed by Professor Horne to assess patients’ perceptions of the intrusiveness of antiretroviral 5 therapies (HAART; the HAART intrusiveness scale).1 This scale assesses convenience and the degree 6 to which the regimen is perceived by the patient to interfere with daily living, social life, etc. The 7 HAART intrusiveness scale has been applied to study differential effects of once- vs. twice-daily 8 antiretroviral regimens2 and might be usefully applied to identify patients who are most likely to 9 benefit from once-daily treatments. 10 11 References: 12 1. Newell, A., Mendes da Costa, S. & Horne, R. Assessing the psychological and therapy-related 13 barriers to optimal adherence: an observational study. Presented at the Sixth International 14 congress on Drug Therapy in HIV Infection, Glasgow, UK (2002). 15 2. Cooper, V., Horne, R., Gellaitry, G., Vrijens, B., Lange, A. C., Fisher, M. et al. The impact of once- 16 nightly versus twice-daily dosing and baseline beliefs about HAART on adherence to efavirenz- 17 based HAART over 48 weeks: the NOCTE study. J Acquir Immune Defic Syndr 53, 369–377 18 (2010). 19 1 20 Supplementary Table S1. Asthma medications, reported by participants at the time of survey Asthma medication n (%) Salbutamol 406 (40.2) Beclometasone 212 (21.0) Salmeterol plus fluticasone 209 (20.7) Salbutamol plus ipratropium 169 (16.7) Formoterol plus budesonide 166