Estimating Density of Secretive Terrestrial Birds (Siamese Fireback) in Pristine and Degraded Forest Using Camera Traps and Distance Sampling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Keeping Pheasants
KKKEEEEEEPPPIIINNNGGG PPPHHHEEEAAASSSAAANNNTTTSSS AAASSS AAA HHHOOOBBBBBBYYY Text and photos: Jan Willem Schrijvers Photo above: Siamese Fireback pheasant male(Lophura diardi). ORIGIN AND LIFESTYLE OF THE WILD PHEASANT Pheasants are wild gallinaceous birds all originating in Asia. One exception is the Congo peacock from Africa. (The pheasants also include game fowl and peacocks.) Each species has its own characteristics and life habits. There are species that live in tropical rain forests, but there are species that live in the mountains, on cold plains. This is something to take in account when housing our pheasants (with or without a night coop, with or without heating). Most species live in and around the mountains with a woodland vegetation, where they can find lots of berries, greens and seeds. It is of utmost importance to first consider the habits and living conditions of the species you want to keep. Only with proper housing will these beautiful birds show to their fullest, and reproduce. PHEASANTS IN AVIARIES In the past there were some "pheasant farms" which mainly bred the common pheasant (also known as ring-neck pheasant). The birds on these farms were bred for sport hunting. They were released en masse in the autumn in the fields and other areas to be shot for the sport. Some escaped from the hunters and that is why today we can see wild pheasants roam our fields and woods. These pheasants have also succeeded in reproducing themselves, even though they are not native birds. The birds are also kept for their colourful feathers. Each year I see Prince Carnival walk again with the beautiful feathers of the Reeves's Pheasant at his cocked hat. -

Hybridization & Zoogeographic Patterns in Pheasants
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Paul Johnsgard Collection Papers in the Biological Sciences 1983 Hybridization & Zoogeographic Patterns in Pheasants Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/johnsgard Part of the Ornithology Commons Johnsgard, Paul A., "Hybridization & Zoogeographic Patterns in Pheasants" (1983). Paul Johnsgard Collection. 17. https://digitalcommons.unl.edu/johnsgard/17 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Paul Johnsgard Collection by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. HYBRIDIZATION & ZOOGEOGRAPHIC PATTERNS IN PHEASANTS PAUL A. JOHNSGARD The purpose of this paper is to infonn members of the W.P.A. of an unusual scientific use of the extent and significance of hybridization among pheasants (tribe Phasianini in the proposed classification of Johnsgard~ 1973). This has occasionally occurred naturally, as for example between such locally sympatric species pairs as the kalij (Lophura leucol11elana) and the silver pheasant (L. nycthelnera), but usually occurs "'accidentally" in captive birds, especially in the absence of conspecific mates. Rarely has it been specifically planned for scientific purposes, such as for obtaining genetic, morphological, or biochemical information on hybrid haemoglobins (Brush. 1967), trans ferins (Crozier, 1967), or immunoelectrophoretic comparisons of blood sera (Sato, Ishi and HiraI, 1967). The literature has been summarized by Gray (1958), Delacour (1977), and Rutgers and Norris (1970). Some of these alleged hybrids, especially those not involving other Galliformes, were inadequately doculnented, and in a few cases such as a supposed hybrid between domestic fowl (Gallus gal/us) and the lyrebird (Menura novaehollandiae) can be discounted. -

Bird Checklists of the World Country Or Region: Myanmar
Avibase Page 1of 30 Col Location Date Start time Duration Distance Avibase - Bird Checklists of the World 1 Country or region: Myanmar 2 Number of species: 1088 3 Number of endemics: 5 4 Number of breeding endemics: 0 5 Number of introduced species: 1 6 7 8 9 10 Recommended citation: Lepage, D. 2021. Checklist of the birds of Myanmar. Avibase, the world bird database. Retrieved from .https://avibase.bsc-eoc.org/checklist.jsp?lang=EN®ion=mm [23/09/2021]. Make your observations count! Submit your data to ebird. -
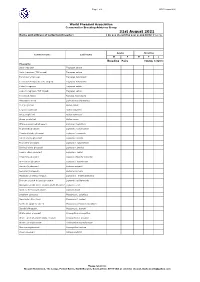
31St August 2021 Name and Address of Collection/Breeder: Do You Closed Ring Your Young Birds? Yes / No
Page 1 of 3 WPA Census 2021 World Pheasant Association Conservation Breeding Advisory Group 31st August 2021 Name and address of collection/breeder: Do you closed ring your young birds? Yes / No Adults Juveniles Common name Latin name M F M F ? Breeding Pairs YOUNG 12 MTH+ Pheasants Satyr tragopan Tragopan satyra Satyr tragopan (TRS ringed) Tragopan satyra Temminck's tragopan Tragopan temminckii Temminck's tragopan (TRT ringed) Tragopan temminckii Cabot's tragopan Tragopan caboti Cabot's tragopan (TRT ringed) Tragopan caboti Koklass pheasant Pucrasia macrolopha Himalayan monal Lophophorus impeyanus Red junglefowl Gallus gallus Ceylon junglefowl Gallus lafayettei Grey junglefowl Gallus sonneratii Green junglefowl Gallus varius White-crested kalij pheasant Lophura l. hamiltoni Nepal Kalij pheasant Lophura l. leucomelana Crawfurd's kalij pheasant Lophura l. crawfurdi Lineated kalij pheasant Lophura l. lineata True silver pheasant Lophura n. nycthemera Berlioz’s silver pheasant Lophura n. berliozi Lewis’s silver pheasant Lophura n. lewisi Edwards's pheasant Lophura edwardsi edwardsi Vietnamese pheasant Lophura e. hatinhensis Swinhoe's pheasant Lophura swinhoii Salvadori's pheasant Lophura inornata Malaysian crestless fireback Lophura e. erythrophthalma Bornean crested fireback pheasant Lophura i. ignita/nobilis Malaysian crestless fireback/Vieillot's Pheasant Lophura i. rufa Siamese fireback pheasant Lophura diardi Southern Cavcasus Phasianus C. colchicus Manchurian Ring Neck Phasianus C. pallasi Northern Japanese Green Phasianus versicolor -

Restricted Bird Species List221.04 KB
RESTRICTED BIRD LICENCE CATEGORIES Exempt Birds - If these species are the only birds kept, no permit is required Canary, Common, Serinus canaria Ground-dove, White-bibbed; Pigeon, White- Pigeon, Domestic; Rock Dove, Columba livia breasted Ground; Jobi Island Dove, Gallicolumba jobiensis Cardinal, Red-crested, Paroaria coronata Guineafowl, Helmeted, Numida meleagris Pigeon, Luzon Bleeding Heart, Gallicolumba luzonica Chicken; Domestic Fowl; all bantams; Red Jungle Fowl, Parrotfinch, Red-throated; Red-faced Parrotfinch, Pytilia, Crimson-winged; Aurora Finch, Pytilia Gallus gallus Erythrura psittacea phoenicoptera Duck, domestic breeds only, Anas spp. Peafowl, Common; Indian Peafowl, Pavo cristatus Pytilia, Green-winged; Melba Finch, Pytilia melba Duck, Mallard; Mallard, Anas platyrhynchos Peafowl, Green, Pavo muticus Swan, Mute; White Swan, Cygnus olor Duck, Muscovy, Cairina moschata Pheasant, Golden, Chrysolophus pictus Turkey, Common, Meleagris gallopavo Firefinch, Red-billed, Lagonosticta senegala Pheasant, Himalayan Monal; Impeyan Pheasant, Turtle-Dove, Laughing, Streptopelia senegalensis Lophophorus impejanus Goldfinch; European Goldfinch, Carduelis carduelis Pheasant, Kalij, Lophura leucomelanos Turtle-Dove, Spotted, Streptopelia chinensis Goose, All Domestic Strains, Anser anser Pheasant, Lady Amherst's, Chrysolophus Waxbill, Lavender; Lavender Finch, Estrilda amherstiae caerulescens Goose, Swan; Chinese Goose, Anser cygnoides Pheasant, Reeves's, Syrmaticus reevesii Waxbill, Zebra; Golden-breasted Waxbill; Orange- breasted Waxbill, -

Gamebird Conservation
Threatened Ducks and Geese West Indian Whistling Duck Hawaiian Goose Gamebird Conservation Freckled Duck by Jack Clinton-Eitniear Crested Shelduck San Antonio, Texas Baykal Teal New Zealand Brown Teal Laysan Duck Pink-headed Duck Madagascar Pochard During the first half of the nine from the list at the end of this article, Scaly-sided Merganser teenth century, had you ventured into they have a rather formidable chal Lesser White-fronted Goose a meat market in ew York or Balti lenge ahead of them. Red-breasted Goose more, you might well have encoun While it is encouraging to note that Ruddy-headed Goose tered a rather "fishy" tasting duck a number of species facing troubles in White-winged Duck offered for sale. That duck, often said the wild are well represented in cap Madagascar Teal to have rotted as few desired to eat tivity it is equally saddening that Hawaiian Duck them, was that of a Labrador Duck most are not. Having had the pleasure Marbled Teal (Camptorhynchus labradorius)~ a of working with tragopans, as well as Baer's Pochard now extinct species. While dis brown-, blue- and white-eared pheas Brazilian Merganser covered inhabiting the northeast sea ants in the late sixties, I know well of White-headed Duck board in 1789, the duck, for reasons their awe-inspiring beauty. Anyone unknown, had disappeared by 1878. who is tempted to associate bright Threatened Pheasants, While at least four species of water colors of enchanting combinations Francolins, Quail & Peafowl fowl have passed into extinction, I with only small birds needs to visit a Bearded Wood-partridge know of only one Gallinaceous bird, pheasant collection. -

MF2310 Resource Guide for Owners of Small Poultry Flocks
Resource Guide for Owners of Small Poultry Flocks R. Scott Beyer • Poultry Specialist Many people are interested in raising small poultry or gamebird flocks for eggs, meat, hobbies, profit or 4-H projects. A small flock is usually inexpensive, requires little space and doesn’t require much time. Raising a small flock is also a good way to introduce youth to food production and build a sense of responsibility. However, as the commercial poultry industry has expanded and consolidated, small flock enthusiasts have had increasing difficulty locating reliable sources of chicks and supplies. The following list of hatcheries and supply stores may not be all inclusive, but should be a good starting point for persons interested in producing poultry and gamebirds on a small scale. This resource guide has been made available to assist small flock owners with locating sources of chicks, waterfowl, gamebirds, peafowl, pigeons and places to purchase equipment supplies from Kansas producers and others. Kansas Hatcheries Web site: www.elkcreekgamebirds.com/pages/5/ E-mail: [email protected] Ark Valley Pheasant Hatchery Friend Bird Farm 9095 South Hutton Road Route 3, Box 24 Ingalls, KS 67853 Fredonia, KS 66736 Phone: 620-335-5399 Phone: 620-378-3946 Beavers’ Game Farm Contact: Kari Friend Hatching Season: 10406 U.S. Highway 50 March to August Breeds Offered: Pierceville, KS 67868 Peafowl: white, blackshoulders, blue Phone: 620-335-5564/5645 Smith Poultry Supply E-mail: [email protected] 14000 W. 215th St. Blue Valley Gamebird Farm Bucyrus, KS 66013 Phone: 1002 Harvest Road 913-879-2587 Fax: 913-533-2497 Marysville, KS 66508 Contact: Phone: John or Terry Smith 785-562-5258 Breeds Offered: Contact: Does not sell chicks or eggs. -

2Xotlc ~Heagahtg 3
2xotlc ~heagahtg 3. Adequate nutritious diet. Most of the species listed as endan eaptiVe/PltVu1.7ement iV1d ~'to?a7t1tion gered or threatened are receivin.g attention in captivity and success IS by Mickey Dllson, Glendale, AZ being attained with many of these. (Aug/Sept 1979 ) ;11iLket{ OliSOl1 has dOl1e mO'le There are 16 genera comprising 48 Edwards' Pheasant The Edwards' Pheasant Lophura nJo'lk 011 pheasMts Md othe'l tjAlLi recognized species of pheasants. In some species there are numerous sub edwardsi is a small and beautiful bird 11aceous 6i'lds thM almost t:lJ1lfol1e species or races bringing the number first discovered in 1895 with a small else i11 Ame'l£CM atJ£cultu'le. d-le of forms to about 150. distribution in central Vietnam near Hue (Way). It was first imported in has alJValfs 6eel1 a J1JiLLiJ1'j tea.che'l, All of the pheasants, including peafowl and junglefowl, are found in 1925 to France by Dr. Jean Delacour, Sha'li115 his k110J1Jl£d5e a60ut these Asia except the Congo Peacock of when he brought some 15 individuals 6i'lds and he J1Jas a pwnee'l in 'lep'lO Africa. to his collection. It was successfully propagated and distributed to zoos ~nd duci115 mAAlf o~ the mO'le di~~£cuLt During the past 50 years radical changes have taken place in much of aviculturists in Europe and Amenca. species. Asia which have severely decimated But recently it has become increasingly wild populations of many species of difficult to breed and abnormalities in he pheasants are among pheasants. -

Central and Northern Thailand Custom Tour Trip Report
CENTRAL AND NORTHERN THAILAND CUSTOM TOUR TRIP REPORT 24 FEBRUARY – 15 MARCH 2019 By Andy Walker We saw a couple of Banded Kingfishers during the tour, fantastic-looking birds. www.birdingecotours.com [email protected] 2 | TRIP REPORT Thailand: Central and North, Feb-Mar 2019 Overview This three-week custom birdwatching tour of Central and Northern Thailand commenced in Bangkok on the 24th of February 2019 and ended back there on the 15th of March 2019. The tour was based on our Central and Northern Thailand set-departure itineraries with a few slight modifications to fit the timescale allowed. We birded areas around the Gulf of Thailand for shorebirds and Kaeng Krachan and Khao Yai National Parks in the central region before flying north for a birding circuit from and back to Chiang Mai, which included visits to Doi Inthanon and Doi Pha Hom Pok National Parks (Doi Lang and Doi Ang Khang), Chiang Dao Wildlife Sanctuary, and Chiang Saen Lake. We also visited a couple of incredible temples along the way. A total of 442 bird species were seen (plus 4 species heard only). Full species lists are provided at the end of this report. Highlight birds seen were many and during our time in the central Thailand region included a couple of Spoon-billed Sandpipers, a large flock of Nordmann’s Greenshanks, and hundreds of Terek Sandpipers on the saltpans among about 40 species of shorebirds, with several ‘White- faced’ Kentish Plovers, Malaysian Plovers, and Chinese Egrets on the nearby beach. A lake and rice fields near the coast hosted Spot-billed Pelican, Asian Dowitcher, Greater Spotted Eagle, and Black-headed Ibis. -

Phasianinae Species Tree
Phasianinae Blood Pheasant,Ithaginis cruentus Ithaginini ?Western Tragopan, Tragopan melanocephalus Satyr Tragopan, Tragopan satyra Blyth’s Tragopan, Tragopan blythii Temminck’s Tragopan, Tragopan temminckii Cabot’s Tragopan, Tragopan caboti Lophophorini ?Snow Partridge, Lerwa lerwa Verreaux’s Monal-Partridge, Tetraophasis obscurus Szechenyi’s Monal-Partridge, Tetraophasis szechenyii Chinese Monal, Lophophorus lhuysii Himalayan Monal, Lophophorus impejanus Sclater’s Monal, Lophophorus sclateri Koklass Pheasant, Pucrasia macrolopha Wild Turkey, Meleagris gallopavo Ocellated Turkey, Meleagris ocellata Tetraonini Ruffed Grouse, Bonasa umbellus Hazel Grouse, Tetrastes bonasia Chinese Grouse, Tetrastes sewerzowi Greater Sage-Grouse / Sage Grouse, Centrocercus urophasianus Gunnison Sage-Grouse / Gunnison Grouse, Centrocercus minimus Dusky Grouse, Dendragapus obscurus Sooty Grouse, Dendragapus fuliginosus Sharp-tailed Grouse, Tympanuchus phasianellus Greater Prairie-Chicken, Tympanuchus cupido Lesser Prairie-Chicken, Tympanuchus pallidicinctus White-tailed Ptarmigan, Lagopus leucura Rock Ptarmigan, Lagopus muta Willow Ptarmigan / Red Grouse, Lagopus lagopus Siberian Grouse, Falcipennis falcipennis Spruce Grouse, Canachites canadensis Western Capercaillie, Tetrao urogallus Black-billed Capercaillie, Tetrao parvirostris Black Grouse, Lyrurus tetrix Caucasian Grouse, Lyrurus mlokosiewiczi Tibetan Partridge, Perdix hodgsoniae Gray Partridge, Perdix perdix Daurian Partridge, Perdix dauurica Reeves’s Pheasant, Syrmaticus reevesii Copper Pheasant, Syrmaticus -

The Status and Conservation of Javan Rhinoceros, Siamese Crocodile, Phasianidae and Gaur in Cat Tien National Park, Vietnam
THE STATUS AND CONSERVATION OF JAVAN RHINOCEROS, SIAMESE CROCODILE, PHASIANIDAE AND GAUR IN CAT TIEN NATIONAL PARK, VIETNAM 2004 BY DAVID MURPHY CAT TIEN NATIONAL PARK CONSERVATION PROJECT TECHNICAL REPORT NO. 50 MAY 2004 THE STATUS AND CONSERVATION OF JAVAN RHINOCEROS, SIAMESE CROCODILE, PHASIANIDAE AND GAUR IN CAT TIEN NATIONAL PARK, VIETNAM 2004 BY DAVID MURPHY CAT TIEN NATIONAL PARK CONSERVATION PROJECT TECHNICAL REPORT NO. 50 MAY 2004 The Cat Tien National Park Conservation Project is a joint initiative of the Ministry of Agriculture and Rural Development (Hanoi) and the WWF- Indochina Programme, funded by the Governments of Vietnam and The Netherlands. 2 Executive Summary Cat Tien National Park is home to a number of species of high conservation value, amongst which the Javan Rhinoceros (Rhinoceros sondaicus annamiticus), Siamese Crocodile (Crocodylus siamensis), rare Phasianidae species (pheasants and partridges), and Gaur (Bos gaurus) are of the highest priority. To expand the information on their populations, on which conservation management decisions are based, the Cat Tien National Park Conservation Project has implemented surveys and established monitoring programmes since 1999. Ongoing research on the Javan Rhino has helped to develop a picture of an animal more critically endangered then previously thought. Current estimates of the population are 2-6, with a possible estimate of 3 individuals remaining in their small range in southern Cat Loc. Of equal concern is the absence of any breeding signs for the last 7 years, and though the presence of a female has been confirmed it is not clear if there are any males. The chances of increasing the population are very marginal and will require larger areas of undisturbed habitat and the presence of male and female animals. -

Robert A.Atkins& Manontentij
The Orange-necked Partridge Arborophila davidi and five other galliforms in two protected areas in southern Vietnam Robert A. Atkins & Manon Tentij The Orange-necked Partridge Arborophila davidi and five other galliforms in two protected areas in southern Vietnam Robert A. Atkins &Manon Tentij August 1998 Institute of Systematics and Population Biology, University of Amsterdam, P.O. Box 94766, 1090 GT, Amsterdam, The Netherlands. Citation: Atkins, R.A. and Tentij, M. 1998. The Orange-necked Partridge Arborophila davidi and five other galliforms in two protected areas in southern Vietnam. Institute of Systematics and Population Biology, University of Amsterdam. 42 p. Atkins & Tentij Table of contents SUMMARY 2 ACKNOWLEDGEMENTS 3 PREFACE 4 1. INTRODUCTION 5 2. LOCATIONS 7 2.1 Cat Tien National Park 7 2.2 Cat Loc Nature Reserve 8 3. METHODS 9 3.1 Survey methods 9 3.1.1 Orange-necked Partridge survey in Cat Tien National Park 9 3.1.2 Orange-necked Partridge survey in Cat Loc Nature Reserve 9 3.1.3 Green Peafowl 9 3.1.4 Line transects 9 3.2 Data analysis 10 4. RESULTS 12 4.1 Orange-necked Partridge 12 4.2 Green Peafowl 13 4.3 Line transect results 13 4.3.1 Siamese Fireback 13 4.3.2 Germain's Peacock-Pheasant 14 4.3.3 Scaly-breasted Partridge 15 4.3.4 Red Junglefowl 17 5. DISCUSSION 18 5.1 Orange-necked Partridge 18 5.2 Census 18 5.3 Threats to Cat Tien National Park 19 5.4 Threats to Cat Loc Nature Reserve 21 6.