Spatial Distribution of Display Sites of Grey Peacock-Pheasant in Relation to Micro-Habitat and Predators During the Breeding Se
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Keeping Pheasants
KKKEEEEEEPPPIIINNNGGG PPPHHHEEEAAASSSAAANNNTTTSSS AAASSS AAA HHHOOOBBBBBBYYY Text and photos: Jan Willem Schrijvers Photo above: Siamese Fireback pheasant male(Lophura diardi). ORIGIN AND LIFESTYLE OF THE WILD PHEASANT Pheasants are wild gallinaceous birds all originating in Asia. One exception is the Congo peacock from Africa. (The pheasants also include game fowl and peacocks.) Each species has its own characteristics and life habits. There are species that live in tropical rain forests, but there are species that live in the mountains, on cold plains. This is something to take in account when housing our pheasants (with or without a night coop, with or without heating). Most species live in and around the mountains with a woodland vegetation, where they can find lots of berries, greens and seeds. It is of utmost importance to first consider the habits and living conditions of the species you want to keep. Only with proper housing will these beautiful birds show to their fullest, and reproduce. PHEASANTS IN AVIARIES In the past there were some "pheasant farms" which mainly bred the common pheasant (also known as ring-neck pheasant). The birds on these farms were bred for sport hunting. They were released en masse in the autumn in the fields and other areas to be shot for the sport. Some escaped from the hunters and that is why today we can see wild pheasants roam our fields and woods. These pheasants have also succeeded in reproducing themselves, even though they are not native birds. The birds are also kept for their colourful feathers. Each year I see Prince Carnival walk again with the beautiful feathers of the Reeves's Pheasant at his cocked hat. -
A Grassland Conservation Plan for Prairie Grouse
A GRASSLAND CONSERVATION PLAN FOR PRAIRIE GROUSE Photo credit: Michael Schroeder Photo credit: Rick Baetsen Photo Credit: Tom Harvey North American Grouse Partnership 2008 Preferred Citation: Vodehnal, W.L., and J.B. Haufler, Editors. 2008. A grassland conservation plan for prairie grouse. North American Grouse Partnership. Fruita, CO. Page i A GRASSLAND CONSERVATION PLAN FOR PRAIRIE GROUSE 2008 STEERING COMMITTEE William L. Vodehnal (Coordinator), Nebraska Game and Parks Commission Rick Baydack, University of Manitoba Dawn M. Davis, University of Idaho Jonathan B. Haufler, Ph.D., Ecosystem Management Research Institute Rob Manes, Kansas-The Nature Conservancy Stephanie Manes, United States Fish and Wildlife Service James A. Mosher, Ph.D., North American Grouse Partnership Steven P. Riley, Nebraska Game and Parks Commission Heather Whitlaw, Texas Parks and Wildlife Department EXECUTIVE SUMMARY Prairie grouse, including all species of prairie-chicken and the sharp-tailed grouse, have declined precipitously and steadily from historical levels throughout the Great Plains of North America. While many factors have contributed to these declines, the loss and fragmentation of expansive prairies to farming, and the reduction of habitat quality within remaining prairie fragments are known to be the primary causes. The social, political and economic drivers that have facilitated this loss of native grasslands throughout the United States and Canada generally fall beyond the jurisdiction of individual local, regional, state, and provincial wildlife management authorities. As a result, many grassland- dependent species requiring high-quality native grasslands are now threatened, endangered, or species of concern. Grasslands have been identified as some of the most endangered ecosystems in North America, so it is not surprising that many associated species are of concern for their level of decline. -

Estimating Density of Secretive Terrestrial Birds (Siamese Fireback) in Pristine and Degraded Forest Using Camera Traps and Distance Sampling
Global Ecology and Conservation xx (xxxx) xxx–xxx Contents lists available at ScienceDirect Global Ecology and Conservation journal homepage: www.elsevier.com/locate/gecco Original research article Estimating density of secretive terrestrial birds (siamese fireback) in pristine and degraded forest using camera traps and distance sampling a,b,∗ b c Q1 Saranphat Suwanrat , Dusit Ngoprasert , Christopher Sutherland , Pongthep Suwanwaree a, Tommaso Savini b a School of Biology, Institute of Science, Suranaree University of Technology, Nakhon Ratchasima, Thailand b Conservation Ecology Program, King Mongkut's University of Technology Thonburi, Bangkok, Thailand c Department of Natural Resources, Cornell University, Ithaca, United States article info a b s t r a c t Article history: Tropical Asian Galliformes are secretive and difficult to survey. Many of these species Received 29 October 2014 are considered ``at risk'' due to habitat degradation although reliable density estimates Received in revised form 28 January 2015 are lacking. Using camera trapping and distance sampling data collected on the Siamese Accepted 28 January 2015 Fireback (Lophura diardi) in northeastern Thailand, we compared density estimates for Available online xxxx pristine and degraded lowland forest. Density was poorly estimated using distance sampling, likely due to small sample size arising from poor visibility in dense vegetation Keywords: and bird's sensitivity to observers. We analyzed camera trap data using both count-based Royle–Nichols model Binomial mixture model and presence/absence-based methods. Those density estimates had narrower confidence Beta-binomial mixture model intervals than those obtained using distance sampling. Estimated density was higher in dry −2 −2 Lophura diardi evergreen forest (5:6 birds km ), than in old forest plantations (0:2 birds km ), perhaps Galliformes because dense forest habitats provide Firebacks with more resources and refuge from Sakaerat Environmental Research Station predation. -

Hybridization & Zoogeographic Patterns in Pheasants
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Paul Johnsgard Collection Papers in the Biological Sciences 1983 Hybridization & Zoogeographic Patterns in Pheasants Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/johnsgard Part of the Ornithology Commons Johnsgard, Paul A., "Hybridization & Zoogeographic Patterns in Pheasants" (1983). Paul Johnsgard Collection. 17. https://digitalcommons.unl.edu/johnsgard/17 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Paul Johnsgard Collection by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. HYBRIDIZATION & ZOOGEOGRAPHIC PATTERNS IN PHEASANTS PAUL A. JOHNSGARD The purpose of this paper is to infonn members of the W.P.A. of an unusual scientific use of the extent and significance of hybridization among pheasants (tribe Phasianini in the proposed classification of Johnsgard~ 1973). This has occasionally occurred naturally, as for example between such locally sympatric species pairs as the kalij (Lophura leucol11elana) and the silver pheasant (L. nycthelnera), but usually occurs "'accidentally" in captive birds, especially in the absence of conspecific mates. Rarely has it been specifically planned for scientific purposes, such as for obtaining genetic, morphological, or biochemical information on hybrid haemoglobins (Brush. 1967), trans ferins (Crozier, 1967), or immunoelectrophoretic comparisons of blood sera (Sato, Ishi and HiraI, 1967). The literature has been summarized by Gray (1958), Delacour (1977), and Rutgers and Norris (1970). Some of these alleged hybrids, especially those not involving other Galliformes, were inadequately doculnented, and in a few cases such as a supposed hybrid between domestic fowl (Gallus gal/us) and the lyrebird (Menura novaehollandiae) can be discounted. -

Bird Checklists of the World Country Or Region: Myanmar
Avibase Page 1of 30 Col Location Date Start time Duration Distance Avibase - Bird Checklists of the World 1 Country or region: Myanmar 2 Number of species: 1088 3 Number of endemics: 5 4 Number of breeding endemics: 0 5 Number of introduced species: 1 6 7 8 9 10 Recommended citation: Lepage, D. 2021. Checklist of the birds of Myanmar. Avibase, the world bird database. Retrieved from .https://avibase.bsc-eoc.org/checklist.jsp?lang=EN®ion=mm [23/09/2021]. Make your observations count! Submit your data to ebird. -

(Syrmaticus Reevesii) Lysozyme
Agric. Biol. Chem., 55 (7), 1707-1713, 1991 1707 The AminoAcid Sequence of Reeves' Pheasant (Syrmaticus reevesii) Lysozyme Tomohiro Araki,* MayumiKuramoto and Takao Torikata Laboratory of Biochemistry, Faculty of Agriculture, Kyushu Tokai University, Aso, Kumamoto 869-14, Japan Received November 13, 1990 The amino acid sequence of reeves' pheasant lysozyme was analyzed. Carboxymethylated lysozyme was digested with trypsin and resulting peptides were analyzed using the DABITC/PITCdouble coupling manual Edmanmethod. The established amino acid sequence had seven substitutions, Tyr3, Leul5, His41, His77, Ser79, ArglO2, and Asnl21, compared with hen egg-white lysozyme. Ser79 was the first found in a bird lysozyme. A substitution in the active site was found in position 102 which has been considered to participate in the substrate binding at subsites A-C. Lysozymeis one of the most characterized and concluded that the enhancement of the hydrolases, which cleave /M,4 linkage of transglycosylation was correlated with the TV-acetylglucosamine (GlcNAc) homopolymer binding of substrate at subsite A. The substrate or GlcNAc-7V-acetylmuramic acid hetero- binding at subsite A has further been polymer. This enzyme is composed of 129-130 investigated by an NMR study.6) The co- amino acids, and the tertiary structure of hen valently bound glucosamine on AsplOl at egg-white lysozyme (HEWL) has been eluci- subsite Acaused a change of the indole proton dated by X-ray refraction study.1} The of Trp62. This result strongly suggested that mechanism of catalytic reaction of this protein the amino acid at position 101 participates in has also been elucidated in detail. The enzyme the interaction between the substrate and contains six substrate binding subsites (A-F) substrate binding site of lysozyme. -

Lesser Prairie-Chicken Initiative Report
Lesser Prairie-Chicken Conservation Beyond Boundaries Initiative Report February 2012 Background/ Did You Know? • The lesser prairie-chicken is a Purpose ground-nesting bird native to the rangelands of the south central Lesser prairie-chicken populations have plains of the U.S. and best declined dramatically during the past several known for its unique courtship decades due to loss of native prairie, habitat displays and “booming” sounds. fragmentation, and degradation of habitat on both private and public lands. The • A lek is an area where lesser Natural Resources Conservation Service prairie-chicken males display (NRCS), its partners and cooperating during the breeding season to landowners are working to increase the number and the range of the lesser attract females; may also be prairie-chicken through the Lesser Prairie-Chicken Initiative (LPCI). At the referred to as a booming ground same time, the initiative is promoting the overall health of grazing lands and or strutting ground. the long-term sustainability of ranching operations.The U.S. Fish and Wildlife Service (USFWS) has worked cooperatively with NRCS to ensure the initiative’s • The lesser prairie-chicken is conservation practices provide long-term benefits to the overall lesser prairie- comparable in morphology, chicken population; minimize or eliminate short-term harmful effects to those plumage and behavior to the populations, and cause no negative effects to other listed and candidate species greater prairie-chicken, although that share habitat with the lesser prairie-chicken. the lesser prairie-chicken is smaller and has distinctive NRCS State Conservationists in Colorado, Kansas, New Mexico, Oklahoma courtship displays and and Texas (states within the lesser prairie-chicken’s range), with the assistance vocalizations. -

Copper Pheasant
Bird Research News Vol.3 No.11 2006.11.10. Last Revised:2013.11.28. Copper Pheasant Yamadori (Jpn) Syrmaticus soemmerringii Morphology and classification bly smaller white part in the boundary zone between the two sub- species ranges (Kawaji 2004). Confusion between the two subspe- cies poses a problem to game hunters and government officials Classification: Galliformes Phasianidae concerned every year because ijimae has been removed from game Wing length: ♂ 205-230mm ♀ 192-219mm bird list since 1979 due to the population decline. Since subspecies Tail length: ♂ 415-952mm ♀ 164-205mm classification could become a major issue in conservation and Culmen length: ♂ 23-31mm ♀ 22-26mm social scenes in the future, genetic analyses are currently underway Tarsus length: ♂ 57-69mm ♀ 53-60mm (Sakanashi et al. 2006). Weight: ♂ 943-1348g ♀ 745-1000g Habitat: All measurement values are of subspecies Syrmaticus soemmerringii scintillans after Kiyosu (1978). Copper Pheasants are a forest dweller, generally preferring a broad -leaved forest. But they also occur in conifer plantations, such as Appearance: Japanese cedar and cypress. They occasionally bathe in the sun Plumage colors of Copper Pheasants and dust in grasslands of a cutover. vary between subspecies. In general, however, male is reddish brown on the Life history upper and underparts with black, chest- nut, yellow and white lateral bars in the 123456789101112 long tail (Photo 1). In the subspecies of breeding season non-breeding season the southern Japan (S.s. soemmerringii and ijimae), the plumage is generally Breeding system: darker with dark red brown and black Copper Pheasants are assumed to be polygamous like Common horizontal bars in the tail. -

CHAPTER 5 PRODUCTS of ANIMAL ORIGIN, NOT ELSEWHERE SPECIFIED OR INCLUDED I 5-1 Notes: 1
)&f1y3X CHAPTER 5 PRODUCTS OF ANIMAL ORIGIN, NOT ELSEWHERE SPECIFIED OR INCLUDED I 5-1 Notes: 1. This chapter does not cover: (a) Edible products (other than guts, bladders and stomachs of animals, whole and pieces thereof, and animal blood, liquid or dried); (b) Hides or skins (including furskins) other than goods of heading 0505 and parings and simlar waste of raw hides or skins of heading 0511 (chapter 41 or 43); (c) Animal textile materials, other than horsehair and horsehair waste (section XI); or (d) Prepared knots or tufts for broom or brush making (heading 9603). 2. For the purposes of heading 0501, the sorting of hair by length (provided the root ends and tip ends, respectively, are not arranged together) shall be deemed not to constitute working. 3. Throughout the tariff schedule, elephant, walrus, narwhal and wild boar tusks, rhinoceros horns and the teeth of all animals are regarded as "ivory." 4. Throughout the tariff schedule, the expression "horsehair" means hair of the manes or tails of equine or bovine animals. Additional U.S. Note 1. (a) Except as provided in paragraphs (b) and (c) of this note, the importation of the feathers or skin of any bird is hereby prohibited. Such prohibition shall apply to the feathers or skin of any bird: (i) Whether raw or processed; (ii) Whether the whole plumage or skin or any part of either; (iii) Whether or not attached to a whole bird or any part thereof; and (iv) Whether or not forming part of another article. (b) Paragraph (a) shall not apply: (i) In respect of any of the following birds (other than any such bird which, whether or not raised in captivity, is a wild bird): chickens (including hens and roosters), turkeys, guineas, geese, ducks, pigeons, ostriches, rheas, English ring-necked pheasants and pea fowl; (ii) To any importation for scientific or educational purposes; (iii) To the importation of fully manufactured artificial flies used for fishing; (iv) To the importation of birds which are classifiable under subheading 9804.00.55; and (v) To the importation of live birds. -

Alpha Codes for 2168 Bird Species (And 113 Non-Species Taxa) in Accordance with the 62Nd AOU Supplement (2021), Sorted Taxonomically
Four-letter (English Name) and Six-letter (Scientific Name) Alpha Codes for 2168 Bird Species (and 113 Non-Species Taxa) in accordance with the 62nd AOU Supplement (2021), sorted taxonomically Prepared by Peter Pyle and David F. DeSante The Institute for Bird Populations www.birdpop.org ENGLISH NAME 4-LETTER CODE SCIENTIFIC NAME 6-LETTER CODE Highland Tinamou HITI Nothocercus bonapartei NOTBON Great Tinamou GRTI Tinamus major TINMAJ Little Tinamou LITI Crypturellus soui CRYSOU Thicket Tinamou THTI Crypturellus cinnamomeus CRYCIN Slaty-breasted Tinamou SBTI Crypturellus boucardi CRYBOU Choco Tinamou CHTI Crypturellus kerriae CRYKER White-faced Whistling-Duck WFWD Dendrocygna viduata DENVID Black-bellied Whistling-Duck BBWD Dendrocygna autumnalis DENAUT West Indian Whistling-Duck WIWD Dendrocygna arborea DENARB Fulvous Whistling-Duck FUWD Dendrocygna bicolor DENBIC Emperor Goose EMGO Anser canagicus ANSCAN Snow Goose SNGO Anser caerulescens ANSCAE + Lesser Snow Goose White-morph LSGW Anser caerulescens caerulescens ANSCCA + Lesser Snow Goose Intermediate-morph LSGI Anser caerulescens caerulescens ANSCCA + Lesser Snow Goose Blue-morph LSGB Anser caerulescens caerulescens ANSCCA + Greater Snow Goose White-morph GSGW Anser caerulescens atlantica ANSCAT + Greater Snow Goose Intermediate-morph GSGI Anser caerulescens atlantica ANSCAT + Greater Snow Goose Blue-morph GSGB Anser caerulescens atlantica ANSCAT + Snow X Ross's Goose Hybrid SRGH Anser caerulescens x rossii ANSCAR + Snow/Ross's Goose SRGO Anser caerulescens/rossii ANSCRO Ross's Goose -

Phylogenetic Relationships of the Phasianidae Reveals Possible Non-Pheasant Taxa
Journal of Heredity 2003:94(6):472–489 Ó 2003 The American Genetic Association DOI: 10.1093/jhered/esg092 Phylogenetic Relationships of the Phasianidae Reveals Possible Non-Pheasant Taxa K. L. BUSH AND C. STROBECK From the Department of Biological Sciences, University of Alberta–Edmonton, Edmonton, Alberta T6G 2E9, Canada. Address correspondence to Krista Bush at the address above, or e-mail: [email protected]. Abstract The phylogenetic relationships of 21 pheasant and 6 non-pheasant species were determined using nucleotide sequences from the mitochondrial cytochrome b gene. Maximum parsimony and maximum likelihood analysis were used to try to resolve the phylogenetic relationships within Phasianidae. Both the degree of resolution and strength of support are improved over previous studies due to the testing of a number of species from multiple pheasant genera, but several major ambiguities persist. Polyplectron bicalcaratum (grey peacock pheasant) is shown not to be a pheasant. Alternatively, it appears ancestral to either the partridges or peafowl. Pucrasia macrolopha macrolopha (koklass) and Gallus gallus (red jungle fowl) both emerge as non-pheasant genera. Monophyly of the pheasant group is challenged if Pucrasia macrolopha macrolopha and Gallus gallus are considered to be pheasants. The placement of Catreus wallichii (cheer) within the pheasants also remains undetermined, as does the cause for the great sequence divergence in Chrysolophus pictus obscurus (black-throated golden). These results suggest that alterations in taxonomic -
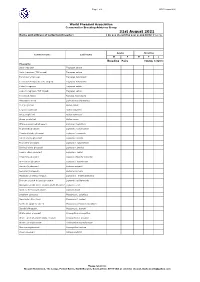
31St August 2021 Name and Address of Collection/Breeder: Do You Closed Ring Your Young Birds? Yes / No
Page 1 of 3 WPA Census 2021 World Pheasant Association Conservation Breeding Advisory Group 31st August 2021 Name and address of collection/breeder: Do you closed ring your young birds? Yes / No Adults Juveniles Common name Latin name M F M F ? Breeding Pairs YOUNG 12 MTH+ Pheasants Satyr tragopan Tragopan satyra Satyr tragopan (TRS ringed) Tragopan satyra Temminck's tragopan Tragopan temminckii Temminck's tragopan (TRT ringed) Tragopan temminckii Cabot's tragopan Tragopan caboti Cabot's tragopan (TRT ringed) Tragopan caboti Koklass pheasant Pucrasia macrolopha Himalayan monal Lophophorus impeyanus Red junglefowl Gallus gallus Ceylon junglefowl Gallus lafayettei Grey junglefowl Gallus sonneratii Green junglefowl Gallus varius White-crested kalij pheasant Lophura l. hamiltoni Nepal Kalij pheasant Lophura l. leucomelana Crawfurd's kalij pheasant Lophura l. crawfurdi Lineated kalij pheasant Lophura l. lineata True silver pheasant Lophura n. nycthemera Berlioz’s silver pheasant Lophura n. berliozi Lewis’s silver pheasant Lophura n. lewisi Edwards's pheasant Lophura edwardsi edwardsi Vietnamese pheasant Lophura e. hatinhensis Swinhoe's pheasant Lophura swinhoii Salvadori's pheasant Lophura inornata Malaysian crestless fireback Lophura e. erythrophthalma Bornean crested fireback pheasant Lophura i. ignita/nobilis Malaysian crestless fireback/Vieillot's Pheasant Lophura i. rufa Siamese fireback pheasant Lophura diardi Southern Cavcasus Phasianus C. colchicus Manchurian Ring Neck Phasianus C. pallasi Northern Japanese Green Phasianus versicolor