Ice Nucleation Activity in the Widespread Soil Fungus Mortierella Alpina” by J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Archaeological Survey of Newton County: Enhancement of a Data Deficient Region, Part II Grant # 18-15FFY-05
An Archaeological Survey of Newton County: Enhancement of a Data Deficient Region, Part II Grant # 18-15FFY-05 By: Jamie M. Leeuwrik, Christine Thompson, and Kevin C. Nolan Principal Investigators: Christine Thompson and Kevin C. Nolan Reports of Investigation 92 Volume 1 May 2016 Applied Anthropology Laboratories, Department of Anthropology Ball State University, Muncie, IN 47306-0439 Phone: 765-285-5328 Fax: 765-285-2163 Web Address: http://www.bsu.edu/aal i An Archaeological Survey of Newton County: Enhancement of a Data Deficient Region, Part II Grant # 18-15FFY-05 By: Jamie M. Leeuwrik, Christine Thompson, and Kevin C. Nolan Christine Thompson and Kevin C. Nolan Principal Investigators ________________________________ Reports of Investigation 92 Volume 1 May 2016 Applied Anthropology Laboratories, Department of Anthropology Ball State University, Muncie, IN 47306-0439 Phone: 765-285-5328 Fax: 765-285-2163 Web Address: http://www.bsu.edu/aal ii ACKNOWLEDGEMENT OF STATE AND FEDERAL ASSISTANCE This project has been funded in part by a grant from the U.S. Department of the Interior, National Park Service’s Historic Preservation Fund administered by the Indiana Department of Natural Resources, Division of Historic Preservation and Archaeology. The project received federal financial assistance for the identification, protection, and/or rehabilitation of historic properties and cultural resources in the State of Indiana. However, the contents and opinions contained in this publication do not necessarily reflect the views or policies of the U.S. Department of the Interior, nor does the mention of trade names or commercial products constitute endorsement or recommendation by the U.S. Department of the Interior. -

MULTICENTENNIAL CLIMATIC CHANGES in the TERE-Khol
Olga K. Borisova1*, Andrei V. Panin1,2 1 Institute of Geography, Russian Academy of Sciences, Moscow, Russia 2 Lomonosov Moscow State University, Moscow, Russia 02|2019 * Corresponding author: [email protected] GES Multicentennial Climatic CHANGES 148 IN THE TERE-KHOL BASIN, SOUTHERN SIBERIA, DURING THE Late Holocene Abstract. Pollen analysis was carried out on an 80-cm sedimentary section on the shore of Lake Tere-Khol (southeastern Tuva). The section consists of peat overlapping lake loams and covers the last 2800 years. The alternation of dry-wet and cold-warm epochs has been established, and changes in heat and moisture occurred non-simultaneously. The first half of the studied interval, from 2.8 to 1.35 kyr BP was relatively arid and warmer on average. Against this background, temperature fluctuations occurred: relatively cold intervals 2.8– 2.6 and 2.05–1.7 kyr BP and relatively warm 2.6-2.05 and 1.7-1.35 kyr BP. The next time interval 1.35-0.7 kyr BP was relatively humid. Against this background, the temperatures varied from cold 1.35-1.1 kyr BP to relatively warm 1.1–0.7 kyr BP. The last 700 years have been relatively cold with a short warming from 400 to 250 years ago. This period included a relatively dry interval 700–400 years ago and more humid climate in the last 400 years. The established climate variability largely corresponds to other climate reconstructions in the Altai-Sayan region. The general cooling trend corresponds to an astronomically determined trend towards a decrease in solar radiation in temperate latitudes of the Northern Hemisphere, and the centennial temperature fluctuations detected against this background correspond well to changes in solar activity reconstructed from 14C production and the concentration of cosmogenic isotopes in Greenland ice. -
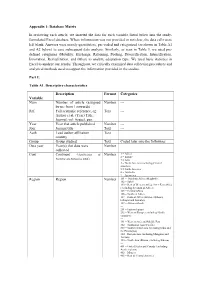
Appendix 1: Database Matrix
Appendix 1: Database Matrix In reviewing each article, we inserted the data for each variable listed below into the ready- formulated Excel database. Where information was not provided or not clear, the data cells were left blank. Answers were mostly quantitative, pre-coded and categorized (as shown in Table A1 and A2 below) to ease subsequent data analysis. Similarly, as seen in Table 3, we used pre- defined categories (Mobility, Exchange, Rationing, Pooling, Diversification, Intensification, Innovation, Revitalization, and Other) to analyze adaptation type. We used basic statistics in Excel to analyze our results. Throughout, we critically examined data collection procedures and analytical methods used to support the information provided in the studies. Part I: Table A1. Descriptive characteristics Description Format Categories Variable Num Number of article (assigned Number --- by us: from 1 onwards) Ref Full scientific reference, eg: Text --- Author et al. (Year) Title, Journal, vol: (issue), pgs. Year Year that article published Number --- Jour Journal title Text --- Auth Lead author affiliation Text country Group Group studied Text Coded later into the following: Data year Year(s) that data were Number collected Cont Continent (classification of Number 1 = Africa 2 = Europe Encyclopedia Britannica 2006) 3 = Asia 4 = North America (including Central America) 5 = South America 6 = Australia 7 = Antarctica Region Region Number 101 = Northern Africa (Maghreb) 102 = Sahel 103 = Rest of Western Africa 104 = East Africa (excluding the Horn -

Pliocene Climates: Scenario for Global Warming
Department of the Interior U.S. Geological Survey Pliocene Climates: Scenario for Global Warming Abstracts from USGS Workshop, Denver, Colorado, October 23 - 25,1989 Edited by Linda B. Gosnell and Richard Z. Poore U.S. Geological Survey, Reston, VA 22092 Open-File Report 90-64 This report is preliminary and has not been reviewed for conformity with U.S. Geological Survey editorial standards and stratigraphic nomenclature. Introduction: USGS Workshop on Pliocene Climates The US. Geological Survey (USGS) held a work tate quantitative estimates of environmental informa shop on Pliocene climates in Denver, Colorado on tion and the development of regional and global pat October 23-25,1989. The workshop brought together terns of climate data. members of the USGS who are working on a long- Better understanding of Pliocene climates, their ev term project to understand and map Pliocene cli olution, and rates and causes of change will provide mates and environments of the Northern Hemi important dues to future earth systems changes and sphere with interested collaborators from the USSR, impacts that will occur as a result of a greenhouse- The Geological Survey of Canada, the National Cen effect global warming. A major goal of the USGS Pli ter for Atmospheric Research, and the Institute for ocene Project is to produce a synoptic map or "snap Arctic and Alpine Research, University of Colorado. shot" of climate parameters during a time in the Plio Paleoclimate researchers from the USSR attended the cene representing conditions that are significantly workshop as part of an exchange and cooperative warmer than modern climates. The Pliocene "Scenar study of Pliocene climates that is organized under the io for Global Warming" will provide a means for test auspices of Working Group VIII of the US-USSR Bi ing and validating results of general circulation mod lateral Agreement on Protection of the Environment els (GCMs) which attempt to model global warming. -

Terrain Intelligence
DEPARTMENT OF THE ARMY FIELD MANUAL TERRAIN INTELLIGENCE HEADQUARTERS, DEPARTMENT OF THE -ARIMY' OCTOBER 1959 FM 30-10 FIELD MANUAL HEADQUARTERS, DEPARTMENT OF THE ARMY No. 30-10 I WASIINQhTON 25, D.C., 28 October 1959 TERRAIN INTELLIGENCE PART ONE. NATURE OF TERRAIN INTELLIGENCE Paragraphs CHAPTER 1. GENERAL.-.................. 1-3 2. INTRODUCTION TO TERRAIN INTELLI- GENCE Section I. Nature of terrain intelligence -............. 4-8 IL Responsibilities for terrain intelligence - 9-12 CHAPTER 3. PRODUCTION OF TERRAIN INTELLI- GENCE Section I. Intelligence cycle .-.................... 13-17 II. Sources and agencies .-................ 18-23 CHAPTER 4. TERRAIN STUDIES Section I. General -..................... 24 29 . II. Basic components of terrain and climate ------- 30-32 III. Describing military aspects of terrain - . 33-43...... IV. Coastal hydrography -................ 44,45 PART TWO. BASIC ELEMENTS OF TERRAIN INFOR- MATION CHAPTER 5. WEATHER AND CLIMATE Section I. Weather -............................... 46-57 II. Climate-............................ 58-64 III. Operational aspects of extreme climates - . 65-67.. CHAPTER 6. NATURAL TERRAIN FEATURES Section I. General -......... 68, 69 II. Landforms --.---------------..--- 70-77 III. Drainage -......................... 78-85 IV. Nearshore oceanography ----------------- 86-91 V. Surface materials -......................... 92, 93 VI. Vegetation .-............................. 94-100 CHAPTER 7. MANMADE TERRAIN FEATURES Section I. General -----------------.------- 101, 102 II. Routes of -

Diptera: Culicidae) in Europe and Its Relationship to the Occurrence of Mosquito-Borne Arboviruses
Acta Zoologica Academiae Scientiarum Hungaricae 65(3), pp. 299–322, 2019 DOI: 10.17109/AZH.65.3.299.2019 EXPLORATION OF THE MAIN TYPES OF BIOME-SCALE CULICID ENTOMOFAUNA (DIPTERA: CULICIDAE) IN EUROPE AND ITS RELATIONSHIP TO THE OCCURRENCE OF MOSQUITO-BORNE ARBOVIRUSES Attila J. Trájer1 and Judit Padisák1,2 1University of Pannonia, Department of Limnology, H-8200 Veszprém, Egyetem u. 10, Hungary E-mail: [email protected]; https://orcid.org/0000-0003-3248-6474 2MTA-PE, Limnoecology Research Group, H-8200 Veszprém, Egyetem u. 10, Hungary E-mail: [email protected]; https://orcid.org/0000-0001-8285-2896 The investigation of the zoogeographical patterns of mosquito faunae and the transmitted arboviruses is an important task in the time of climate change. We aimed to characterize the possibly existing large-scale mosquito faunae in Europe and compare to the occurrence of mosquito-borne arboviruses. The zoogeography of 100 mosquito taxa was investigated in a country and territory-level distribution. Based on the result of hierarchical clustering, four main large-scale faunae were found in Europe: a Mediterranean, a transitional-insu- lar, a continental and a boreal. Significant differences were found between the taxonomic compositions of the faunae in genus level. Climatic classes have no significant influence on the number of mosquito species of an area in Europe, but each of the faunae has climazonal range. The results revealed that Culiseta and Ochlerotatus species, those are less implicated in the transmission of human pathogenic agents, are characteristic to the mosquito fauna of the more humid and cold climate areas. -

Historical Climate and Climate Trends in the Midwestern USA
Historical Climate and Climate Trends in the Midwestern USA WHITE PAPER PREPARED FOR THE U.S. GLOBAL CHANGE RESEARCH PROGRAM NATIONAL CLIMATE ASSESSMENT MIDWEST TECHNICAL INPUT REPORT Jeff Andresen1,2, Steve Hilberg3, and Ken Kunkel4 1 Michigan State Climatologist 2 Michigan State University 3 Midwest Regional Climate Center 4 Desert Research Institute Recommended Citation: Andresen, J., S. Hilberg, K. Kunkel, 2012: Historical Climate and Climate Trends in the Midwestern USA. In: U.S. National Climate Assessment Midwest Technical Input Report. J. Winkler, J. Andresen, J. Hatfield, D. Bidwell, and D. Brown, coordinators. Available from the Great Lakes Integrated Sciences and Assessments (GLISA) Center, http://glisa.msu.edu/docs/NCA/MTIT_Historical.pdf. At the request of the U.S. Global Change Research Program, the Great Lakes Integrated Sciences and Assessments Center (GLISA) and the National Laboratory for Agriculture and the Environment formed a Midwest regional team to provide technical input to the National Climate Assessment (NCA). In March 2012, the team submitted their report to the NCA Development and Advisory Committee. This white paper is one chapter from the report, focusing on potential impacts, vulnerabilities, and adaptation options to climate variability and change for the historical climate sector. U.S. National Climate Assessment: Midwest Technical Input Report: Historical Climate Sector White Paper Contents Introduction ................................................................................................................................................................................................... -

North American Geology
DEPARTMENT OF THE INTERIOR Hubert Work, Secretary U. S. GEOLOGICAL SURVEY George Otis Smith, Director Bulletin 802 BIBLIOGRAPHY OF NORTH AMERICAN GEOLOGY FOR 1925 AND 1926 BY JOHN M. NICKLES UNITED STATES GOVERNMENT PRINTING OFFICE WASHINGTON 1928 ADDITIONAL COPIES OF THIS PUBLICATION MAT BE PROCURED FROM THE SUPERINTENDENT OF DOCUMENTS U. S. GOVERNMENT PRINTING OFFICE WASHINGTON, D. C. AT 40 CENTS PER COPY CONTENTS Page Introduction. __ ______!____ _ _____________. 1 Serials examined_______________________________.. 3 Bibliography____________________________________ 9 Index______________________________________. 187 Lists________________________________________. 274 Chemical analyses______________________ ___ . 274 Mineral analyses_______________i_________________ . 276 Minerals described_____________________________. 276 Rocks described _______ _ . 278 Geologic formations described . 279 in BIBLIOGRAPHY OF NORTH AMERICAN GEOLOGY FOE 1925 AND 1926 By JOHN M. NICKLES INTRODUCTION The bibliography of North American geology, including paleon tology, petrology, and mineralogy, for the years 1925 and 1926 con tains publications on the geology of the Continent of North America and adjacent islands and on Panama and the Hawaiian Islands. It includes textbooks and papers of general character by American au thors, but not those by foreign authors, except papers that appear in American publications. The papers, with full title and medium of publication are listed under the names of their authors, which are arranged in alphabetic order. The author list is followed by an index to the literature listed. The bibliography of North American geology is comprised in the following bulletins of the United States Geological Survey: No. 127 (1732-1892); Nos. 188 and 189 (1892-1900); No. 301 (1901-1905); No. 372 (1906-7); No. 409 (1908); No. 444 (1909); No. 495 (1910); No. -

An Examination of Microthermal Structure Statistics As Calculated from Expendable Bathythermograph Records
N EXAMINATION OF im / (in; "RMAL STRUCTURE •JaiM -•• MmBm59W£W» Igfa rag &s& & <&\ *o*' United States Naval Postgraduate School m SCttOOU y w THESIS AN EXAMINATION OF MICROTHERMAL STRUCTURE STATISTICS AS CALCULATED FROM EXPENDABLE BATHYTHERMOGRAPH RECORDS by Frank Martin Hunt , Jr. // Thesis Advisor W.W. Denner September 1971 Approved {oh pub tic hc.le.aiZ) dLiVu.buJU.ovi unlimLtzd. An Examination of Microthermal Structure Statistics as Calculated from Expendable Bathythermograph Records by Frank Martin Hunt , Jr. Lieutenant Commander, United States Navy B.S. , United States Naval Academy, 1960 Submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE IN OCEANOGRAPHY from the NAVAL POSTGRADUATE SCHOOL September 1971 ABSTRACT Expendable Bathythermograph (XBT) temperature depth profile data from 17 locations in the north Pacific Ocean has been analyzed to extract information concerning the thermal microstructure. The microstructure information is then examined to determine if the distribution can be associated with the climatic regions of the north Pacific Ocean proposed by Tully [ 1964 ]. A modified binomial filter used by Roden [ 1971 ] to analyze STD data was applied to find a mean temperature series. A temperature per- turbation series results when the mean is subtracted f rom the original XBT series. Two resulting parameters , average mean temperature perturbation vertical thickness scale and average maximum amplitude of the temperature perturbations , can be associated with the micro- structure. The average mean temperature perturbation vertical thickness scale was found to increase with latitude from 20°N to 55°N , and as the thick- ness scale increased, the maximum amplitude of the perturbations was found to decrease. -

Regional Pattern of Climates in Europe According to the Thornthwaite Classification*
REGIONAL PATTERN OF CLIMATES IN EUROPE ACCORDING TO THE THORNTHWAITE CLASSIFICATION* JACK RICHARD VILLMOW Department of Geography, The Ohio State University, Columbus 10 INTRODUCTION Over ten years have elapsed since Thornthwaite (1948) published his revised classification of climates. Since that date, when the arrangement of climatic types in the United States was first published, a wide variety of studies have appeared concerning other areas: Canada (Sanderson, 1948), Turkey (Erinc, 1950), New Zealand (Gamier, 1951), Argentina (Burgos and Vidal, 1951), Rhodesias and Nyasaland (Howe, 1953), China (Chang, 1955), and others. More recently an evaluation of the revised classification of climates appeared in the Annals of the Association of American Geographers (Chang, 1959). Chang notes the need for the development of a better energy budget formula to take into account the more significant role of radiation than air temperature in promoting evapotranspiration. Until the physicist or the meteorologist achieves this goal the geographer can continue to exploit the positive values of the classifica- tion: a better understanding of the macrovariations in climate over the earth's surface; the establishing of correlations or interrelationships between climatic elements (e.g., Thornthwaite's Moisture Index and the specialized procedure for calculating soil moisture) and other elements of the physical environment as a basis for understanding economic patterns of land use. This study examines the application of the 1948 Thornthwaite classification of climates to Europe, including the western part of the Soviet Union, and proceeds to compare the arrangement of climates on that continent with the pattern observed in the United States. OBSERVATIONAL DATA Blue Hill Meteorological Observatory in Milton, Massachusetts, recently completed a systematic revision of the time-honored volume of climatic data, Clayton's World Weather Records, and has made available a reasonably complete and hitherto unused series of climatic data for the European landmass. -

NW Asia According to Pollen Records in Three Offshore Cores from the Black
Late Quaternary vegetation and climate of SE Europe - NW Asia according to pollen records in three offshore cores from the Black and Marmara seas Speranta - Maria Popescu, Gonzalo Jimenez-Moreno, Stefan Klotz, Gilles Lericolais, François Guichard, M. Namik Cagatay, Liviu Giosan, Michel Calleja, Séverine Fauquette, Jean-Pierre Suc To cite this version: Speranta - Maria Popescu, Gonzalo Jimenez-Moreno, Stefan Klotz, Gilles Lericolais, François Guichard, et al.. Late Quaternary vegetation and climate of SE Europe - NW Asia according to pollen records in three offshore cores from the Black and Marmara seas. Palaeobiodiversity and Palaeoenvironments, Springer Verlag, In press. hal-03104560 HAL Id: hal-03104560 https://hal.archives-ouvertes.fr/hal-03104560 Submitted on 9 Jan 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Palaeobiodiversity and Palaeoenvironments 2020 Doi: 10.1007/s12549-020-00464-x Late Quaternary vegetation and climate of SE Europe – NW Asia according to pollen records in three offshore cores from the Black and Marmara seas Speranta-Maria Popescu1*, Gonzalo Jiménez-Moreno2, Stefan Klotz3, Gilles Lericolais4, François Guichard5, M. Namık Çağatay6, Liviu Giosan7, Michel Calleja8, Séverine Fauquette9, Jean-Pierre Suc10 1, GeoBioStratData.Consulting, 385 route du Mas Rillier, 69140 Rillieux la Pape, France ([email protected]). -

"Paleoecolog of Two Peat Deposits on the Oregon Coast"
Paleoecology ofTwo Peat Deposits on the Oregon Coast BY HENRY P. HANSEN, Ph.D. INSTRUCTOR IN BOTANY OREGON STATE COLLEGE CORVALLIS, OREGON OREGON STATE MONOGRAPHS Studies in Botany Number 3, May 20, 1941 Published by Oregon State College Oregon State System of Higher Education Corvallis, Oregon PREFACE This is one in a series of pollen studies of post-Pleistocene peat and other pollen-bearing sediments in the Pacific Northwest. Bogs in British Columbia, northern Idaho, many parts of Washington, and in west central Oregon have been analyzed and the forest succession interpreted from the pollen profiles. A tentative climatic trend has also been suggested. It has been shown that most of the bogs had their origin soon after the recession of the last continental ice sheet, and thus record most of the postglacial forest succession in adjacent regions. This study is the second of Oregon peat deposits, and the author expects to make many more analyses in Oregon and other parts of the Pacific Northwest. Eventually, a correlation will be made of most of the pollen profiles and a picture of post-Pleistocene forest succes sion and climate reconstructed for the entire region. Many of these peat deposits lie within different climax vegetation areas. This fact should aid in the interpretation of specific fluctuations and in the evaluation of the pollen profiles. The existence of the same species of forest trees in several climax regions with different climates should help to clarify the meaning of their pollen profile fluctuations rather than to confuse them. The expenses of collecting the field data and of the materials used in preparing the peat for microscopic analysis were defrayed by a grant-in-aid from the General Research Council of the Oregon State System of Higher Education.