Diptera: Culicidae) in Europe and Its Relationship to the Occurrence of Mosquito-Borne Arboviruses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acuerdos Y Convenios
Ministerio de Relaciones Exteriores Instrumentos Bilaterales suscritos entre la República del Paraguay y la República del Ecuador FIRMA RATIFICACION PARAGUAY FECHA N° NOMBRE DEL DOCUMENTO ENTRADA EN LUGAR FECHA Nº LEY FECHA VIGOR 1. Tratado de Exención de Derechos de Visa. Panamá 3/X/1939 --- --- NO VIGENTE Acuerdo para la Supresión de Visas de los Pasaportes Diplomáticos, Quito 13/VII/1967 --- --- 12/VIII/1967 2. Oficiales o de Servicio y Comunes. 3. Convenio de Intercambio Cultural. Quito 28/VI/1968 64 26/XII/1968 8/VIII/1969 4. Acuerdo para el Intercambio de Valijas Diplomáticas. Quito 18/I/1971 -- -- 18/I/1971 5. Acuerdo sobre reciprocidad de radioaficionados. N.R. N° 6. Asunción 25/VII/1985 Dto. 11.856 24/IX/1985 24/II/1986 Acuerdo que Establece un Mecanismo de Consulta entre los Asunción 14/VIII/1991 14/VIII/1991 6. Ministerios de Relaciones Exteriores. 7. Programa de Intercambio Cultural Paraguayo – Ecuatoriano Asunción 14/VIII/91 --- --- --- 8. Acuerdo de Cooperación entre las Academias Diplomáticas. Quito 02/VI/1993 --- --- 02/VI/1993 Ministerio de Relaciones Exteriores Acuerdo de Cooperación entre los Ministerios de Relaciones Quito 02/VI/1993 --- --- NO VIGENTE 9. Exteriores. 10. Declaración Conjunta de Cancilleres. Quito 02/VI/1993 11. Convenio Básico de Cooperación Económica, Científica y Técnica. Quito 02/VI/1993 242 10/IX/1993 9/VIII/1999 NO VIGENTE 12. Convenio sobre Promoción y Protección Recíproca de Inversiones. Quito 28/I/1994 469 14/XI/1994 Denunciado el 18/1/2008 13. Comunicado Conjunto de los señores Presidentes para la Prensa. Quito 4/II/1994 Acuerdo que establece la Comisión Permanente de Coordinación entre Asunción 15/IX/1994 --- --- 15/IX/1994 14. -

Quito Ciudad, Capital Del Ecuador
Quito ciudad, capital del Ecuador Datos generales / General Information Extensión / extension 422.802 hectáreas / hectares Altitud / altitude 2.850 msnm. (9.350 pies / feet) Temperatura ambiental / weather Entre 10 y 25 grados centígrados / 50-77 Farenheit degrees Moneda oficial / currency Dólar estadounidense / U.S. dollar Impuestos / taxes 12% al valor agregado y 10% de servicios turísticos Tarjetas de crédito / credit cards Visa, Mastercard, American Express, Diners Club Electricidad / electricity 110 AC/110V Coordenadas / coordinates -0.1865943,-78.4305382 Quito, la primera ciudad declarada por la UNESCO Patrimonio Cultural de la Humanidad, está situada en la cordillera de los Andes. La ciudad está rodeada de doce volcanes, entre ellos: Pichincha, Cotopaxi, Antisana, Cayambe, que conforman un contorno andino majestuoso. El centro histórico de Quito es probablemente el mayor de América Latina, no sólo por su extensión sino por los monumentos arquitectónicos que contiene. Así, Quito te ofrece una variedad de opciones culturales, académicas, recreativas, museos, librerías y espectáculos. Geografía Quito es la segunda ciudad capital más alta del mundo a 2830 metros sobre el nivel del mar, tiene una superficie de 4.183 km² y alrededor de 2.2 millones de habitantes. La ciudad se ha desarrollado en el eje norte-sur (tiene más de 80 km de largo y 5 km de ancho) y está del lado occidental de la Cordillera de los Andes, en plena mitad del mundo. Se divide en cinco sectores: zona norte, centro norte, centro histórico, sur y valles aledaños. En el centro norte de la ciudad se ubica FLACSO Ecuador. Clima El clima de Quito es variable, con temperaturas que pueden ir de los 25 grados centígrados de día a los 10 grados en la noche y no es extraño que en un mismo día se combinen fuertes lluvias, temperaturas bajas y soles brillantes. -

Living and Learning in Quito, Ecuador
Living and Learning in Quito, Ecuador A Semester Abroad Experience Living and Learning in Quito, Ecuador Semester Abroad Study Program Living and Learning in Quito, Ecuador is a study abroad program available to students who desire to continue their college education in an international setting. Living and Learning in Quito will function under the supervision and guidelines of your North American institution. This 13-week semester is designed to combine classroom learning and practical internships in a unique and challenging cross-cultural setting. Along with receiving up to 16 units of college credit, students will live, learn and grow academically, experientially, and spiritually. The men and women who participate in this program will be asked to consider how they can use their gifts and talents to reach the world with the good news of Jesus Christ. While living in Quito for 13 weeks students will experience a variety of new cultures, gain a global perspective and understand in a new way the joys and the challenges of serving God in a cross-cultural setting. Students will enjoy the rich interaction with teachers, faculty, and ministry site hosts in a classroom setting, as well as on a one-to-one level. We believe this unique opportunity and setting will lend itself to life long impact. If you accept this challenge, you will: • LEARN through Spanish language study and interdisciplinary seminars about Latin culture, history, ecology, politics, economics, and religion. • LEARN through college level courses that apply toward your major • LIVE with Ecuadorian families, improving your Spanish and sharing your life with Latin American Christians. -

Proceso De Quito Hoja De Ruta Del Capítulo De Buenos Aires
IV REUNIÓN TÉCNICA INTERNACIONAL SOBRE MOVILIDAD HUMANA DE NACIONALES VENEZOLANOS- Proceso de Quito Hoja de Ruta del Capítulo de Buenos Aires E~ ocasión de la IV Reunión Técnica Internacional sobre Movilidad Humana, Proceso de Quito -Capítulo de Buenos Aires- celebrada el 4 y 5 de julio en esta ciudad, y en el marco del Plan de Acción de Quito suscrito el 23 de noviembre de 2018, los abajo firmantes acuerdan: l. Evaluar el establecimiento de un grupo de trabajo, integrado por las áreas nacionales competentes en convalidación y/o revalidación de títulos, para intercambiar buenas prácticas y experiencias en la materia que permitan facilitar dichos procesos, conforme a las respectivas normativas nacionales. 2. Valorar positivamente el ofrecimiento de OIM de organizar un taller regional de autoridades en 'Tlateria de trata de personas de los países participantes del Proceso de Quito, para intercamt:>iar buenas prácticas y avances en la región, frente a la problemática específica que wnilev2 21 flujo masivo de nacionales venezolanos. 3. Analizar la propuesta de OPS y ONUSIDA a fin de incrementar la cobertura de atención en ""lud hücia las personas migrantes y refugiadas que viven con VIH" y solicitarles hagan llegar a la Presidencia Pro Tempere del Proceso de Quito, el desarrollo de la misma para su consideración por parte de las áreas competentes en materia de salud de cada país, con miras a continuar su tratamiento en la V Reunión en Colombia. 4. Considerar los perfiles de proyectos de cooperación que se anexan a esta Hoja de Ruta, ~~~~ ~;"" de trabajo para continuar articulando una respuesta coordinada a nivel regional. -

South America Bingo Myfreebingocards.Com
South America Bingo myfreebingocards.com Safety First! Before you print all your bingo cards, please print a test page to check they come out the right size and color. Your bingo cards start on Page 3 of this PDF. If your bingo cards have words then please check the spelling carefully. If you need to make any changes go to mfbc.us/e/kenm7 Play Once you've checked they are printing correctly, print off your bingo cards and start playing! On the next page you will find the "Bingo Caller's Card" - this is used to call the bingo and keep track of which words have been called. Your bingo cards start on Page 3. Virtual Bingo Please do not try to split this PDF into individual bingo cards to send out to players. We have tools on our site to send out links to individual bingo cards. For help go to myfreebingocards.com/virtual-bingo. Help If you're having trouble printing your bingo cards or using the bingo card generator then please go to https://myfreebingocards.com/faq where you will find solutions to most common problems. Share Pin these bingo cards on Pinterest, share on Facebook, or post this link: mfbc.us/s/kenm7 Edit and Create To add more words or make changes to this set of bingo cards go to mfbc.us/e/kenm7 Go to myfreebingocards.com/bingo-card-generator to create a new set of bingo cards. Legal The terms of use for these printable bingo cards can be found at myfreebingocards.com/terms. -

An Archaeological Survey of Newton County: Enhancement of a Data Deficient Region, Part II Grant # 18-15FFY-05
An Archaeological Survey of Newton County: Enhancement of a Data Deficient Region, Part II Grant # 18-15FFY-05 By: Jamie M. Leeuwrik, Christine Thompson, and Kevin C. Nolan Principal Investigators: Christine Thompson and Kevin C. Nolan Reports of Investigation 92 Volume 1 May 2016 Applied Anthropology Laboratories, Department of Anthropology Ball State University, Muncie, IN 47306-0439 Phone: 765-285-5328 Fax: 765-285-2163 Web Address: http://www.bsu.edu/aal i An Archaeological Survey of Newton County: Enhancement of a Data Deficient Region, Part II Grant # 18-15FFY-05 By: Jamie M. Leeuwrik, Christine Thompson, and Kevin C. Nolan Christine Thompson and Kevin C. Nolan Principal Investigators ________________________________ Reports of Investigation 92 Volume 1 May 2016 Applied Anthropology Laboratories, Department of Anthropology Ball State University, Muncie, IN 47306-0439 Phone: 765-285-5328 Fax: 765-285-2163 Web Address: http://www.bsu.edu/aal ii ACKNOWLEDGEMENT OF STATE AND FEDERAL ASSISTANCE This project has been funded in part by a grant from the U.S. Department of the Interior, National Park Service’s Historic Preservation Fund administered by the Indiana Department of Natural Resources, Division of Historic Preservation and Archaeology. The project received federal financial assistance for the identification, protection, and/or rehabilitation of historic properties and cultural resources in the State of Indiana. However, the contents and opinions contained in this publication do not necessarily reflect the views or policies of the U.S. Department of the Interior, nor does the mention of trade names or commercial products constitute endorsement or recommendation by the U.S. Department of the Interior. -

Special Update on Quito Process V Technical Meeting on Human
Special update on Quito Process AS OF NOVEMBER 2019 LATIN AMERICA AND THE CARIBBEAN AS OF NOVEMBER 2019 LATIN AMERICA AND THE CARIBBEAN V Technical Meeting on HumanV EMobilityNEZUVELEANNEZUE LofAN Venezuelan Citizens in the RegionREFUGEERSE F&U MGIEGERSA &N TMSIG INR ATNHTES R IENG TIOHNE REGION UNITED STATES UNITED STATES BOGOTA, COLOMBIA Havana\ MEXICO Havana\ MEXICO CUBA DOMINICAN 14-15 NOVEMBER 2019 CUBA DOMINICAN \ REPUBLIC Mexico \ HAITI \ PUERTO REPUBLIC Mexico JAMAICA \ Santo HAITI \ PUERTO City \BELIZE JAMAICA \ RICO City \ Domingo Santo RICO BELIZE Domingo \ HONDURAS CARIBBEAN SEA GUATEMALA \ \ HONDURAS CARIBBEAN SEA \ GUATEMALA \ EL SALVADOR NICARAGUA \ ARUBA CURACAO \ EL SALVADOR APoRrUt BA \NICARAGUA Spain CURACAO Port San José \ \ TRINIDAD Spain \ Panamá San JoséCaracas & TOBA\GO \ TRINIDAD PARTICIPATION COSTA \ \ Caracas Panamá & TOBAGO COSTA \ RICA PANAMA Georgetown RICA PANAMAVENEZUELA \ Georgetown VENEZ\UPaErLaAmaribo \ GUYANA Paramaribo \Bogotá \ CGayUeYnAneNA \ \Bogotá \ Cayenne COLOMBIA SURINAME COLOMBIA FRENCH SURINAME GUYANA FRENCH The fifth round of the Quito Process (Quito V – GUYANA \Quito \Quito ECUADOR Bogota Chapter) took place on 14-15 November ECUADOR (2019), with the participation of 12 States from PERU Latin America and the Caribbean, along with PERU \Lima BRAZIL key cooperating States, international financial \Lima BRAZIL \ BOLIVIA Brasilia \ BOLIVIA Brasilia institutions and other actors. \ \ Sucre Sucre PACIFIC PARAGUAY ATLANTIC PACIFIC PARAGUAY ATLANTIC OCEAN Asunción \ OCEAN The meeting follows up on four previous meetings OCEAN Asunción \ OCEAN QUITO V DECLARATION in September and November 2018, April and July QUITO V DECLARATION SIGNATORIES ARGENTINA SIGNATORIES ARGENTINA 2019, as part of a government-led initiative thatArge ntina \ URUGUAY Argentina \ URUGUAY Santiago \ \ Santiago \ Brazil Buenos Montevideo \ aims to harmonize the policies and practices of Brazil Aires Buenos Montevideo Chile CHILE Aires Chile CHILE the countries in the region. -

MULTICENTENNIAL CLIMATIC CHANGES in the TERE-Khol
Olga K. Borisova1*, Andrei V. Panin1,2 1 Institute of Geography, Russian Academy of Sciences, Moscow, Russia 2 Lomonosov Moscow State University, Moscow, Russia 02|2019 * Corresponding author: [email protected] GES Multicentennial Climatic CHANGES 148 IN THE TERE-KHOL BASIN, SOUTHERN SIBERIA, DURING THE Late Holocene Abstract. Pollen analysis was carried out on an 80-cm sedimentary section on the shore of Lake Tere-Khol (southeastern Tuva). The section consists of peat overlapping lake loams and covers the last 2800 years. The alternation of dry-wet and cold-warm epochs has been established, and changes in heat and moisture occurred non-simultaneously. The first half of the studied interval, from 2.8 to 1.35 kyr BP was relatively arid and warmer on average. Against this background, temperature fluctuations occurred: relatively cold intervals 2.8– 2.6 and 2.05–1.7 kyr BP and relatively warm 2.6-2.05 and 1.7-1.35 kyr BP. The next time interval 1.35-0.7 kyr BP was relatively humid. Against this background, the temperatures varied from cold 1.35-1.1 kyr BP to relatively warm 1.1–0.7 kyr BP. The last 700 years have been relatively cold with a short warming from 400 to 250 years ago. This period included a relatively dry interval 700–400 years ago and more humid climate in the last 400 years. The established climate variability largely corresponds to other climate reconstructions in the Altai-Sayan region. The general cooling trend corresponds to an astronomically determined trend towards a decrease in solar radiation in temperate latitudes of the Northern Hemisphere, and the centennial temperature fluctuations detected against this background correspond well to changes in solar activity reconstructed from 14C production and the concentration of cosmogenic isotopes in Greenland ice. -
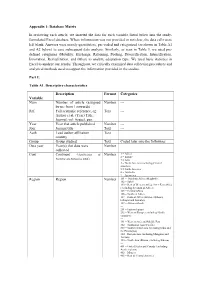
Appendix 1: Database Matrix
Appendix 1: Database Matrix In reviewing each article, we inserted the data for each variable listed below into the ready- formulated Excel database. Where information was not provided or not clear, the data cells were left blank. Answers were mostly quantitative, pre-coded and categorized (as shown in Table A1 and A2 below) to ease subsequent data analysis. Similarly, as seen in Table 3, we used pre- defined categories (Mobility, Exchange, Rationing, Pooling, Diversification, Intensification, Innovation, Revitalization, and Other) to analyze adaptation type. We used basic statistics in Excel to analyze our results. Throughout, we critically examined data collection procedures and analytical methods used to support the information provided in the studies. Part I: Table A1. Descriptive characteristics Description Format Categories Variable Num Number of article (assigned Number --- by us: from 1 onwards) Ref Full scientific reference, eg: Text --- Author et al. (Year) Title, Journal, vol: (issue), pgs. Year Year that article published Number --- Jour Journal title Text --- Auth Lead author affiliation Text country Group Group studied Text Coded later into the following: Data year Year(s) that data were Number collected Cont Continent (classification of Number 1 = Africa 2 = Europe Encyclopedia Britannica 2006) 3 = Asia 4 = North America (including Central America) 5 = South America 6 = Australia 7 = Antarctica Region Region Number 101 = Northern Africa (Maghreb) 102 = Sahel 103 = Rest of Western Africa 104 = East Africa (excluding the Horn -

Christina Park, Brendan Bottger Thank You, Bolivar! Simon Bolivar
Christina Park, Brendan Bottger Thank you, Bolivar! Simon Bolivar 1783 Creole born in Caracas, Venezuela Enlightenment education “[…] symbol and hero of liberation struggle” (Keen 161) John Locke Voltaire Montesquieu General Causes Creoles vs. Peninsulares Different POVs: empire (mercantilism) vs. nativism “Institutionalized discrimination” (Keen) Enlightenment influences French, American Revolutions Free press disseminate ideas General Causes Napoleonic Wars Charles IV and Ferdinand VII imprisonedà juntas ○ Creoles’ dream of self-rule= more realistic (Keen) Want free trade! (with Britain, maybe) 1749 Venezuelan merchant revolt 1781 Comunero Revolt 1793 Consulado de Caracas Development of Revolutions 1. 1810-1814 à Initial start and expansion of movement 2. 1814-1816 à Resurgence of royalist (peninsulares) domination 3. 1817-1824 à Actual independence Opposition in Venezuela 1810 formation of creole-dominated junta 1811 Venezuelan independence Continuing conflict b/t patriots and royalists Patriots lose several battles under Miranda Miranda flees Venezuelaà Bolivar prevents Spain declares terror against all patriots Stirrings in Colombia Bolivar to New Granada (Colombia) Manifesto to the Citizens of New Granada ○ Venezuelan independence=Colombian security (Bolivar) 1813 victory at Cucuta General Bolivar uses guerilla warfare Promotes based on merit To the Rest of Spanish Am. General Bolivar leads 500 men to Caracas Declares counterterror against all Spaniards Spaniards retreat Bolivar triumphant entrance to -

Oil Exploitation in the Yasuní National Park and the Rights of Nature Carlos Larrea
Oil Exploitation in the Yasuní National Park and the Rights of Nature Carlos Larrea Ecuador is one of the most biodiverse countries on the planet, with the largest number of vertebrates per square kilometer in the world . The country is among the top ten international positions by number of amphibians, birds and butterflies, and holds second place for the number of orchids ( Josse, 2001 , Hassler and Rheinheimer , 2013) . Yasuni National Park has been considered the most biodiverse place in the Western Hemisphere . It was created in 1979 and declared a UNESCO World Biosphere Reserve in 1989. It includes 982,000 hectors in the upper Napo basin in the western Amazon. Its strategic location close to the Equator and the Andes mountain range, provides unique climatic conditions in the Amazon, with relatively uniform temperature and high humidity. Scientists agree the only value of the Park is for its extraordinary biodiversity, conservation status and cultural heritage. With 2,274 species of trees and shrubs, the park hosts up to 655 species in a single hectare, most of all native tree species in the United States and Canada. It has 593 species of birds and 80 species of bats, 150 amphibians and 121 reptiles and over 4,000 species of vascular plants per 1,000 km2 hectares. The number of insect species estimated at 100,000 ha, is the largest on the planet. Among these species there is a high degree of endemism. The park has the highest density of species of amphibians, mammals , birds and plants in the Amazon. In addition , the temperature rise due to climate change will be comparatively moderate , giving it a strategic importance for the future conservation of species. -

Pliocene Climates: Scenario for Global Warming
Department of the Interior U.S. Geological Survey Pliocene Climates: Scenario for Global Warming Abstracts from USGS Workshop, Denver, Colorado, October 23 - 25,1989 Edited by Linda B. Gosnell and Richard Z. Poore U.S. Geological Survey, Reston, VA 22092 Open-File Report 90-64 This report is preliminary and has not been reviewed for conformity with U.S. Geological Survey editorial standards and stratigraphic nomenclature. Introduction: USGS Workshop on Pliocene Climates The US. Geological Survey (USGS) held a work tate quantitative estimates of environmental informa shop on Pliocene climates in Denver, Colorado on tion and the development of regional and global pat October 23-25,1989. The workshop brought together terns of climate data. members of the USGS who are working on a long- Better understanding of Pliocene climates, their ev term project to understand and map Pliocene cli olution, and rates and causes of change will provide mates and environments of the Northern Hemi important dues to future earth systems changes and sphere with interested collaborators from the USSR, impacts that will occur as a result of a greenhouse- The Geological Survey of Canada, the National Cen effect global warming. A major goal of the USGS Pli ter for Atmospheric Research, and the Institute for ocene Project is to produce a synoptic map or "snap Arctic and Alpine Research, University of Colorado. shot" of climate parameters during a time in the Plio Paleoclimate researchers from the USSR attended the cene representing conditions that are significantly workshop as part of an exchange and cooperative warmer than modern climates. The Pliocene "Scenar study of Pliocene climates that is organized under the io for Global Warming" will provide a means for test auspices of Working Group VIII of the US-USSR Bi ing and validating results of general circulation mod lateral Agreement on Protection of the Environment els (GCMs) which attempt to model global warming.