Assessing Passeriformes Health in South Texas Via Select Venous Analytes MARK ⁎ J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

6-A John James Audubon, American Flamingo, 1838
JOHN JAMES AUDUBON [1785–1851] 6 a American Flamingo,1838 American Flamingo is one of the 435 hand-colored engravings that River, a major flyway for migratory birds, and eventually wan- make up John James Audubon’s monumental Birds of America, dered farther from home to comb the American frontier for issued in four volumes between 1826 and 1838. The massive unrecorded species. publication includes life-size representations of nearly five hundred Audubon’s procedure was to study and sketch a bird in its natural species of North American birds. Although Audubon was not the habitat before killing it carefully, using fine shot to minimize dam- first to attempt such a comprehensive catalog, his work departed age. His critical innovation was to then thread wire through the from conventional scientific illustration, which showed lifeless spec- specimen, allowing him to fashion a lifelike pose. He worked in imens against a blank background, by presenting the birds as they watercolor, and had completed some four hundred paintings appeared in the wild. When his pictures were first published, when he decided to publish them as a folio of prints. Failing to find some naturalists objected to Audubon’s use of dramatic action and support in Philadelphia, he sailed for England, where he became pictorial design, but these are the qualities that set his work apart lionized as “The American Woodsman.” The engraving firm and make it not only an invaluable record of early American Robert Havell and Son took on the challenge of reproducing wildlife but an unmatched work of American art. Audubon’s paintings on copper plates and tinting the resulting John James Audubon was born in Haiti and educated in France, black-and-white prints by hand. -

Spring in South Texas
SPRING IN SOUTH TEXAS MARCH 31–APRIL 9, 2019 Green Jay, Quinta Mazatlan, McAllen, Texas, April 5, 2019, Barry Zimmer LEADERS: BARRY ZIMMER & JACOB DRUCKER LIST COMPILED BY: BARRY ZIMMER VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM SPRING IN SOUTH TEXAS MARCH 31–APRIL 9, 2019 By Barry Zimmer Once again, our Spring in South Texas tour had it all—virtually every South Texas specialty, wintering Whooping Cranes, plentiful migrants (both passerine and non- passerine), and rarities on several fronts. Our tour began with a brief outing to Tule Lake in north Corpus Christi prior to our first dinner. Almost immediately, we were met with a dozen or so Scissor-tailed Flycatchers lining a fence en route—what a welcoming party! Roseate Spoonbill, Crested Caracara, a very cooperative Long-billed Thrasher, and a group of close Cave Swallows rounded out the highlights. Strong north winds and unsettled weather throughout that day led us to believe that we might be in for big things ahead. The following day was indeed eventful. Although we had no big fallout in terms of numbers of individuals, the variety was excellent. Scouring migrant traps, bays, estuaries, coastal dunes, and other habitats, we tallied an astounding 133 species for the day. A dozen species of warblers included a stunningly yellow male Prothonotary, a very rare Prairie that foraged literally at our feet, two Yellow-throateds at arm’s-length, four Hooded Warblers, and 15 Northern Parulas among others. Tired of fighting headwinds, these birds barely acknowledged our presence, allowing unsurpassed studies. -

Landbird Habitat Conservation Strategy – 2020 Revision
Upper Mississippi / Great Lakes Joint Venture Landbird Habitat Conservation Strategy – 2020 Revision Upper Mississippi / Great Lakes Joint Venture Landbird Habitat Conservation Strategy – 2020 Revision Joint Venture Landbird Committee Members: Kelly VanBeek, U.S. Fish and Wildlife Service (Migratory Birds), Co-chair Chris Tonra, The Ohio State University, Co-chair Mohammed Al-Saffar, U.S. Fish and Wildlife Service (Joint Venture) Dave Ewert, American Bird Conservancy Erin Gnass Giese, University of Wisconsin – Green Bay Allisyn-Marie Gillet, Indiana Dept. of Natural Resources Shawn Graff, American Bird Conservancy Jim Herkert, Illinois Audubon Sarah Kendrick, Missouri Dept. of Conservation Mark Nelson, U.S.D.A. Forest Service Katie O’Brien, U.S. Fish and Wildlife Service (Migratory Birds) Greg Soulliere, U.S. Fish and Wildlife Service (Joint Venture) Wayne Thogmartin, U.S. Geological Survey Mike Ward, University of Illinois at Urbana-Champaign Tom Will, U.S. Fish and Wildlife Service (Migratory Birds) Recommended citation: Soulliere, G. J., M. A. Al-Saffar, K. R. VanBeek, C. M. Tonra, M. D. Nelson, D. N. Ewert, T. Will, W. E. Thogmartin, K. E. O’Brien, S. W. Kendrick, A. M. Gillet, J. R. Herkert, E. E. Gnass Giese, M. P. Ward, and S. Graff. 2020. Upper Mississippi / Great Lakes Joint Venture Landbird Habitat Conservation Strategy – 2020 Revision. U.S. Fish and Wildlife Service, Bloomington, Minnesota, USA. Cover photo: Whip-poor-will, by Ian Sousa-Cole. TABLE OF CONTENTS STRATEGY SUMMARY ..................................................................................................1 -

Flamingo ABOUT the GROUP
Flamingo ABOUT THE GROUP Bulletin of the IUCN-SSC/Wetlands International The Flamingo Specialist Group (FSG) was established in 1978 at Tour du Valat in France, under the leadership of Dr. Alan Johnson, who coordinated the group until 2004 (see profile at www.wetlands.org/networks/Profiles/January.htm). Currently, the group is FLAMINGO SPECIALIST GROUP coordinated from the Wildfowl & Wetlands Trust at Slimbridge, UK, as part of the IUCN- SSC/Wetlands International Waterbird Network. The FSG is a global network of flamingo specialists (both scientists and non- scientists) concerned with the study, monitoring, management and conservation of the world’s six flamingo species populations. Its role is to actively promote flamingo research and conservation worldwide by encouraging information exchange and cooperation amongst these specialists, and with other relevant organisations, particularly IUCN - SSC, Ramsar, WWF International and BirdLife International. FSG members include experts in both in-situ (wild) and ex-situ (captive) flamingo conservation, as well as in fields ranging from field surveys to breeding biology, diseases, tracking movements and data management. There are currently 165 members around the world, from India to Chile, and from France to South Africa. Further information about the FSG, its membership, the membership list serve, or this bulletin can be obtained from Brooks Childress at the address below. Chair Assistant Chair Dr. Brooks Childress Mr. Nigel Jarrett Wildfowl & Wetlands Trust Wildfowl & Wetlands Trust Slimbridge Slimbridge Glos. GL2 7BT, UK Glos. GL2 7BT, UK Tel: +44 (0)1453 860437 Tel: +44 (0)1453 891177 Fax: +44 (0)1453 860437 Fax: +44 (0)1453 890827 [email protected] [email protected] Eastern Hemisphere Chair Western Hemisphere Chair Dr. -

Trinidad & Tobago II 2019 BIRDS
Field Guides Tour Report Trinidad & Tobago II 2019 Dec 27, 2019 to Jan 5, 2020 Tom Johnson For our tour description, itinerary, past triplists, dates, fees, and more, please VISIT OUR TOUR PAGE. Each one of those red dots is a Scarlet Ibis - and look closely, you'll see a strip of pink from the American Flamingo flock at the bottom. This was our evening scene at Caroni Swamp. Photo by group member Delle Daniels. The island nation of Trinidad and Tobago provides an accessible bounty of neotropical wildlife with comfortable accommodation. Our holiday tour this year was no exception – we spent five nights at the famed Asa Wright Nature Centre in Trinidad and two nights on the sandy shores of the Atlantic on Tobago. We drank coffee and tea in the morning as the rising sun brought legions of hummingbirds and honeycreepers to the feeding station below the Asa Wright veranda, studied the mating displays of the odd Bearded Bellbird and transforming White-bearded Manakin, sought the stunning Blue-and- yellow Macaw at Nariva Swamp, enjoyed the waves of brilliant Scarlet Ibis ripple past at Caroni Swamp, and watched with excitement as Magnificent Frigatebirds tail-chased Red-billed Tropicbirds over the blue water below Little Tobago Island. There was something for everyone – for those who were taken by Neotropical birding challenges, there were Chestnut-collared and Lesser Swallow-tailed swifts to pick out of the skies above the Arima Valley and a Gray-throated Leaftosser skulking in the shadows. For those more attracted to spectacles of movement and color, we turned to the diminutive Tufted Coquettes of Asa Wright’s gardens and the jaw-droppingly pink American Flamingos striding below the river of Scarlet Ibis at Caroni Swamp. -

1- Checklist of New Mexico
CHECKLIST OF NEW MEXICO BIRD SPECIES Sartor O. Williams III Secretary, New Mexico Bird Records Committee [email protected] 1 January 2021 This checklist contains all the species of birds that have been verified by specimen, photograph, or audio recording in New Mexico and have been accepted as valid by the New Mexico Bird Records Committee. Nomenclature, taxonomy, sequence, and spelling follow the seventh edition of the American Ornithological Society’s [formerly American Ornithologists’ Union, or AOU] Check-list of North American Birds (1998) as amended through the 61st Supplement (Auk 2020, Vol. 137; 24 pp.). Included are all families and species of birds known to occur (or have occurred) in New Mexico in the historical period (1540 to present). Through 1 January 2021, 549 species representing 68 families have been verified in New Mexico, including five established non-native species (identified as “Introduced”) and three species now extirpated (identified as “Extirpated”). Family ANATIDAE: Ducks, Geese, Swans Black-bellied Whistling-Duck, Dendrocygna autumnalis Fulvous Whistling-Duck, Dendrocygna bicolor Snow Goose, Anser caerulescens Ross’s Goose, Anser rossii Greater White-fronted Goose, Anser albifrons Brant, Branta bernicla Cackling Goose, Branta hutchinsii Canada Goose, Branta canadensis Trumpeter Swan, Cygnus buccinator Tundra Swan, Cygnus columbianus Wood Duck, Aix sponsa Garganey, Spatula querquedula Blue-winged Teal, Spatula discors Cinnamon Teal, Spatula cyanoptera Northern Shoveler, Spatula clypeata Gadwall, Mareca -

Flamingo Newsletter 17, 2009
ABOUT THE GROUP The Flamingo Specialist Group (FSG) is a global network of flamingo specialists (both scientists and non-scientists) concerned with the study, monitoring, management and conservation of the world’s six flamingo species populations. Its role is to actively promote flamingo research, conservation and education worldwide by encouraging information exchange and cooperation among these specialists, and with other relevant organisations, particularly the IUCN Species Survival Commission (SSC), the Ramsar Convention on Wetlands, the Convention on Conservation of Migratory Species (CMS), the African-Eurasian Migratory Waterbird Agreement (AEWA), and BirdLife International. The group is coordinated from the Wildfowl & Wetlands Trust, Slimbridge, UK, as part of the IUCN-SSC/Wetlands International Waterbird Network. FSG members include experts in both in-situ (wild) and ex-situ (captive) flamingo conservation, as well as in fields ranging from research surveys to breeding biology, infectious diseases, toxicology, movement tracking and data management. There are currently 286 members representing 206 organisations around the world, from India to Chile, and from France to South Africa. Further information about the FSG, its membership, the membership list serve, or this bulletin can be obtained from Brooks Childress at the address below. Chair Dr. Brooks Childress Wildfowl & Wetlands Trust Slimbridge Glos. GL2 7BT, UK Tel: +44 (0)1453 860437 Fax: +44 (0)1453 860437 [email protected] Eastern Hemisphere Chair Western Hemisphere Chair Dr. Arnaud Béchet Dr. Felicity Arengo Station biologique, Tour du Valat American Museum of Natural History Le Sambuc Central Park West at 79th Street 13200 Arles, France New York, NY 10024 USA Tel : +33 (0) 4 90 97 20 13 Tel: +1 212 313-7076 Fax : +33 (0) 4 90 97 20 19 Fax: +1 212 769-5292 [email protected] [email protected] Citation: Childress, B., Arengo, F. -

Ecology, Morphology, and Behavior in the New World Wood Warblers
Ecology, Morphology, and Behavior in the New World Wood Warblers A dissertation presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Doctor of Philosophy Brandan L. Gray August 2019 © 2019 Brandan L. Gray. All Rights Reserved. 2 This dissertation titled Ecology, Morphology, and Behavior in the New World Wood Warblers by BRANDAN L. GRAY has been approved for the Department of Biological Sciences and the College of Arts and Sciences by Donald B. Miles Professor of Biological Sciences Florenz Plassmann Dean, College of Arts and Sciences 3 ABSTRACT GRAY, BRANDAN L., Ph.D., August 2019, Biological Sciences Ecology, Morphology, and Behavior in the New World Wood Warblers Director of Dissertation: Donald B. Miles In a rapidly changing world, species are faced with habitat alteration, changing climate and weather patterns, changing community interactions, novel resources, novel dangers, and a host of other natural and anthropogenic challenges. Conservationists endeavor to understand how changing ecology will impact local populations and local communities so efforts and funds can be allocated to those taxa/ecosystems exhibiting the greatest need. Ecological morphological and functional morphological research form the foundation of our understanding of selection-driven morphological evolution. Studies which identify and describe ecomorphological or functional morphological relationships will improve our fundamental understanding of how taxa respond to ecological selective pressures and will improve our ability to identify and conserve those aspects of nature unable to cope with rapid change. The New World wood warblers (family Parulidae) exhibit extensive taxonomic, behavioral, ecological, and morphological variation. -

Federal Register/Vol. 85, No. 74/Thursday, April 16, 2020/Rules
21282 Federal Register / Vol. 85, No. 74 / Thursday, April 16, 2020 / Rules and Regulations DEPARTMENT OF THE INTERIOR United States and the Government of United States or U.S. territories as a Canada Amending the 1916 Convention result of recent taxonomic changes; Fish and Wildlife Service between the United Kingdom and the (8) Change the common (English) United States of America for the names of 43 species to conform to 50 CFR Part 10 Protection of Migratory Birds, Sen. accepted use; and (9) Change the scientific names of 135 [Docket No. FWS–HQ–MB–2018–0047; Treaty Doc. 104–28 (December 14, FXMB 12320900000//201//FF09M29000] 1995); species to conform to accepted use. (2) Mexico: Convention between the The List of Migratory Birds (50 CFR RIN 1018–BC67 United States and Mexico for the 10.13) was last revised on November 1, Protection of Migratory Birds and Game 2013 (78 FR 65844). The amendments in General Provisions; Revised List of this rule were necessitated by nine Migratory Birds Mammals, February 7, 1936, 50 Stat. 1311 (T.S. No. 912), as amended by published supplements to the 7th (1998) AGENCY: Fish and Wildlife Service, Protocol with Mexico amending edition of the American Ornithologists’ Interior. Convention for Protection of Migratory Union (AOU, now recognized as the American Ornithological Society (AOS)) ACTION: Final rule. Birds and Game Mammals, Sen. Treaty Doc. 105–26 (May 5, 1997); Check-list of North American Birds (AOU 2011, AOU 2012, AOU 2013, SUMMARY: We, the U.S. Fish and (3) Japan: Convention between the AOU 2014, AOU 2015, AOU 2016, AOS Wildlife Service (Service), revise the Government of the United States of 2017, AOS 2018, and AOS 2019) and List of Migratory Birds protected by the America and the Government of Japan the 2017 publication of the Clements Migratory Bird Treaty Act (MBTA) by for the Protection of Migratory Birds and Checklist of Birds of the World both adding and removing species. -

Population Size and Movements of the Greater Flamingo (Phoenicopterus Roseus) in the Jaffna Peninsula, Sri Lanka: Results from a Long-Term Study
Ceylon Journal of Science 47(4) 2018: 373-378 DOI: http://doi.org/10.4038/cjs.v47i4.7555 RESEARCH ARTICLE Population size and movements of the Greater Flamingo (Phoenicopterus roseus) in the Jaffna peninsula, Sri Lanka: Results from a long-term study Chaminda S. Wijesundara1,*, Saumya Wanniarachchi1, Tharangi Hettiarachchi1, Supun Galappaththi1, Asela Weerawardhana1 and Packiyanathan Rajkumar2,3 1Department of Zoology, University of Peradeniya, Peradeniya, Sri Lanka 2Postgraduate Institute of Science, University of Peradeniya, Peradeniya, Sri Lanka 3Divisional Secretariat, Chundukkuli, Jaffna, Sri Lanka Received:12/05/2018; Accepted:02/08/2018 Abstract: The Greater Flamingo (Phoenicopterus roseus) is an and western Africa, from east Africa to South Africa and uncommon migrant bird species found in Sri Lanka, and is a major Madagascar, and east to Kazakhstan and through Middle attraction among avitourists. Jaffna Peninsula, Mannar Island, and East to India and Sri Lanka (Primack, 2010; del Hoyo et the southeastern coastal areas are the known strongholds of this al., 2017). In Sri Lanka, it is mainly found in the northern species in Sri Lanka. Previous studies on this species in the Jaffna parts of the island (Wijesundara et al., 2017b), where, in Peninsula are limited, most probably due to the inaccessibility some areas such as Jaffna region, it is one of the most of the area during the three-decade long civil war. Hence, the abundant migratory bird species (Wijesundara et al., objectives of the present study were to determine the population 2016). Even though it is generally recognized as a migrant size and movements of the Greater Flamingo in major flocking species, a large number can be seen year-round in the areas in the Jaffna Peninsula. -

Long-Legged Pink Things
nld n hn: Wht r th? Whr d th fr? DAVID S. LEE N.C. State Museum of Natural Sciences P.O. Box 27647, Raleigh, N.C. 27611 Pearson et al. (1942), Sprunt and Chamberlain (1949), and the American Ornithol- ogists' Union Check-list (1957, 1983) consider the records of Greater [American] Flamin- gos 1 in the Carolinas as naturally occurring vagrants. The primary South Carolina records are ones provided by Audubon (1840-1844) and Wayne (1887). The Audubon record is somewhat vague. "A very few of these birds have been known to proceed eastward of the Floridas beyond Charleston in South Carolina, and some have been procured there within eight or ten years back." Wayne's record is of a young, storm-driven male killed on DeBardien Island in September 1876. The specimen was not saved. Sprunt and Chamber- lain (1949) cite an apparent "tongue in cheek" news clipping from the Charleston Courier on 20 July 1818 providing evidence of an even earlier record. It states, "We hope that they [other migrating birds] will meet with better reception than the unfortunate flamingo who recently paid us the honor of a visit from South America, but before he arrived in the metropolis, was slain at John's Island by a man who mistook him for a British soldier." The news article states that the bird was placed in the Charleston Museum, but by 1949 there was no record of its existence. Other records of flamingos available for South Carolina are provided in Table 1. In North Carolina the earliest record was made by the manager of the Pea Island Refuge, Samuel A. -
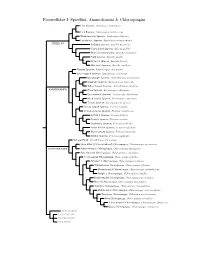
Passerellidae Species Tree
Passerellidae I: Spizellini, Ammodramini & Chlorospingini Lark Sparrow, Chondestes grammacus Lark Bunting, Calamospiza melanocorys Black-throated Sparrow, Amphispiza bilineata Five-striped Sparrow, Amphispiza quinquestriata SPIZELLINI Chipping Sparrow, Spizella passerina Clay-colored Sparrow, Spizella pallida Black-chinned Sparrow, Spizella atrogularis Field Sparrow, Spizella pusilla Brewer’s Sparrow, Spizella breweri Worthen’s Sparrow, Spizella wortheni Tumbes Sparrow, Rhynchospiza stolzmanni Stripe-capped Sparrow, Rhynchospiza strigiceps Grasshopper Sparrow, Ammodramus savannarum Grassland Sparrow, Ammodramus humeralis Yellow-browed Sparrow, Ammodramus aurifrons AMMODRAMINI Olive Sparrow, Arremonops rufivirgatus Green-backed Sparrow, Arremonops chloronotus Black-striped Sparrow, Arremonops conirostris Tocuyo Sparrow, Arremonops tocuyensis Rufous-winged Sparrow, Peucaea carpalis Cinnamon-tailed Sparrow, Peucaea sumichrasti Botteri’s Sparrow, Peucaea botterii Cassin’s Sparrow, Peucaea cassinii Bachman’s Sparrow, Peucaea aestivalis Stripe-headed Sparrow, Peucaea ruficauda Black-chested Sparrow, Peucaea humeralis Bridled Sparrow, Peucaea mystacalis Tanager Finch, Oreothraupis arremonops Short-billed (Yellow-whiskered) Chlorospingus, Chlorospingus parvirostris CHLOROSPINGINI Yellow-throated Chlorospingus, Chlorospingus flavigularis Ashy-throated Chlorospingus, Chlorospingus canigularis Sooty-capped Chlorospingus, Chlorospingus pileatus Wetmore’s Chlorospingus, Chlorospingus wetmorei White-fronted Chlorospingus, Chlorospingus albifrons Brown-headed