Intercellular Calcium Signaling in a Gap Junction-Coupled Cell Network Establishes Asymmetric Neuronal Fates in C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
ION CHANNEL DIVERSITY and CHARACTERIZATION
ION CHANNEL DIVERSITY and CHARACTERIZATION Voltage clamp techniques K channels Na channels Ca channels Ligand-gated channels Channelopathies OUTLINE Voltage clamp techniques whole cell, single channel, gating K channels Na channels Ca channels Cardiac AP Na nerve vs cardiac Ica: L vs T type; drugs (BayK, nitrendipine) Ito: inactivation, subtypes Kv1.4, Kv4.2/3, accessory subunits Ikr,Iks, Ikur (drugs dofetilide) IK1 – rectification Cardiac channelopathies (LQTS, SQTS, Brugada syndrome) Ligand-gated channels (AChR) Pancreatic beta cell channels (KATP, ICa) Voltage clamp techniques Capacitance currents (Ic) and ionic currents (Ii) are activated by rapid changes in membrane potential using voltage clamp Variable Vtest can be applied with voltage clamp 2.0 sec 40 duration 20 (10 sec between each pulse) 0 -20 mV test -40 V -60 -80 Simplified schematic of voltage clamp circuit Original patch clamp recordings (1981) Pflugers Arch 391: 85-100 Four modes of patch clamp technique High-throughput, automated patch clamp instruments EVOLUTION and Ion channel diversity Diversity of ion channels Example: nematode C. elegans 73 K channels (20 6 TM, 3 IRK, 50 TWIK) 89 ligand-gated channels (42 ACh, 37 inhibitory GABAA or glutamate, 10 excitatory glutamate) 5 voltage-gated Ca channels 6 chloride channels 24 gap junction channels (connexins) 22 mechanosensitive channels 6 cyclic-nucleotide gated channels 11 TRP-related channels Total: 236 channel subunit genes Origin of ion channel diversity 1) gene duplication & divergence 2) alternative mRNA splicing 3) -

Electrical Synapses Are Drivers of Neural Plasticity Through Passage of Small Molecules
Electrical Synapses are Drivers of Neural Plasticity Through Passage of Small Molecules Lisa Voelker A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2019 Reading Committee: Jihong Bai, Chair Linda Buck Cecilia Moens Program Authorized to Offer Degree: Molecular and Cellular Biology ©Copyright 2019 Lisa Voelker 2 University of Washington Abstract Electrical Synapses are Drivers of Neural Plasticity through Passage of Small Molecules Lisa Voelker Chair of the Supervisory Committee: Jihong Bai Department of Biochemistry In order to respond to changing environments and fluctuations in internal states, animals adjust their behavior through diverse neuromodulatory mechanisms. In this study we show that electrical synapses between the ASH primary quinine-detecting sensory neurons and the neighboring ASK neurons are required for modulating the aversive response to the bitter tastant quinine in C. elegans. Mutant worms that lack the electrical synapse proteins INX-18 and INX-19 become hypersensitive to dilute quinine. Cell-specific rescue experiments indicate that inx-18 operates in ASK while inx-19 is required in both ASK and ASH for proper quinine sensitivity. Imaging analyses find that INX-19 in ASK and ASH localizes to the same regions in the nerve ring, suggesting that both sides of ASK-ASH electrical synapses contain INX-19. While inx-18 and inx-19 mutant animals have a similar behavioral phenotype, several lines of evidence suggest the proteins encoded by these genes play different roles in modulating the aversive quinine response. First, INX-18 and INX-19 localize 3 to different regions of the nerve ring, indicating that they are not present in the same synapses. -
New Group of Transmembrane Proteins Associated with Desiccation Tolerance in the Anhydrobiotic Midge Polypedilum Vanderplanki Taisiya A
www.nature.com/scientificreports OPEN New group of transmembrane proteins associated with desiccation tolerance in the anhydrobiotic midge Polypedilum vanderplanki Taisiya A. Voronina1,6, Alexander A. Nesmelov1,6, Sabina A. Kondratyeva1, Ruslan M. Deviatiiarov1, Yugo Miyata2, Shoko Tokumoto3, Richard Cornette2, Oleg A. Gusev1,4,5, Takahiro Kikawada2,3* & Elena I. Shagimardanova1* Larvae of the sleeping chironomid Polypedilum vanderplanki are known for their extraordinary ability to survive complete desiccation in an ametabolic state called “anhydrobiosis”. The unique feature of P. vanderplanki genome is the presence of expanded gene clusters associated with anhydrobiosis. While several such clusters represent orthologues of known genes, there is a distinct set of genes unique for P. vanderplanki. These include Lea-Island-Located (LIL) genes with no known orthologues except two of LEA genes of P. vanderplanki, PvLea1 and PvLea3. However, PvLIL proteins lack typical features of LEA such as the state of intrinsic disorder, hydrophilicity and characteristic LEA_4 motif. They possess four to fve transmembrane domains each and we confrmed membrane targeting for three PvLILs. Conserved amino acids in PvLIL are located in transmembrane domains or nearby. PvLEA1 and PvLEA3 proteins are chimeras combining LEA-like parts and transmembrane domains, shared with PvLIL proteins. We have found that PvLil genes are highly upregulated during anhydrobiosis induction both in larvae of P. vanderplanki and P. vanderplanki-derived cultured cell line, Pv11. Thus, PvLil are a new intriguing group of genes that are likely to be associated with anhydrobiosis due to their common origin with some LEA genes and their induction during anhydrobiosis. Anhydrobiosis is the ability of an organism to survive complete desiccation in the ametabolic state. -

Altered Calcium Handling in Cerebellar Purkinje Neurons with the Malignant Hyperthermia Mutation, Ryr1-Y522S/+
University of Denver Digital Commons @ DU Electronic Theses and Dissertations Graduate Studies 1-1-2011 Altered Calcium Handling in Cerebellar Purkinje Neurons with the Malignant Hyperthermia Mutation, RyR1-Y522S/+ George C. Talbott University of Denver Follow this and additional works at: https://digitalcommons.du.edu/etd Part of the Biochemistry, Biophysics, and Structural Biology Commons, and the Biology Commons Recommended Citation Talbott, George C., "Altered Calcium Handling in Cerebellar Purkinje Neurons with the Malignant Hyperthermia Mutation, RyR1-Y522S/+" (2011). Electronic Theses and Dissertations. 638. https://digitalcommons.du.edu/etd/638 This Thesis is brought to you for free and open access by the Graduate Studies at Digital Commons @ DU. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons @ DU. For more information, please contact [email protected],[email protected]. ALTERED CALCIUM HANDLING IN CEREBELLAR PURKINJE NEURONS WITH THE MALIGNANT HYPERTHERMIA MUTATION, RYR1 Y522S/+ __________ A Thesis Presented to The Faculty of Natural Sciences and Mathematics University of Denver __________ In Partial Fulfillment of the Requirements for the Degree Master of Science __________ by George C. Talbott June 2011 Advisor: Nancy M. Lorenzon, PhD ©Copyright by George C. Talbott 2011 All Rights Reserved Author: George C. Talbott Title: ALTERED CALCIUM HANDLING IN CEREBELLAR PURKINJE NEURONS WITH THE MALIGNANT HYPERTHERMIA MUTATION, RYR1 Y522S/+ Advisor: Nancy M. Lorenzon, PhD Degree Date: June 2011 Abstract To investigate the etiology of malignant hyperthermia and central core disease, mouse models have recently been generated and characterized (Chelu et al., 2006). These RyR Y522S/+ knock-in mutant mice provide an excellent tool to investigate calcium dysregulation, its pathological consequences, and potential therapeutic approaches. -

Src Regulation of Cx43 Phosphorylation and Gap Junction Turnover
biomolecules Article Src Regulation of Cx43 Phosphorylation and Gap Junction Turnover Joell L. Solan 1 and Paul D. Lampe 1,2,* 1 Translational Research Program, Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA; [email protected] 2 Department of Global Health, Pathobiology Program, University of Washington, Seattle, WA 98109, USA * Correspondence: [email protected] Received: 27 October 2020; Accepted: 22 November 2020; Published: 24 November 2020 Abstract: The gap junction protein Connexin43 (Cx43) is highly regulated by phosphorylation at over a dozen sites by probably at least as many kinases. This Cx43 “kinome” plays an important role in gap junction assembly and turnover. We sought to gain a better understanding of the interrelationship of these phosphorylation events particularly related to src activation and Cx43 turnover. Using state-of-the-art live imaging methods, specific inhibitors and many phosphorylation-status specific antibodies, we found phospho-specific domains in gap junction plaques and show evidence that multiple pathways of disassembly exist and can be regulated at the cellular and subcellular level. We found Src activation promotes formation of connexisomes (internalized gap junctions) in a process involving ERK-mediated phosphorylation of S279/282. Proteasome inhibition dramatically and rapidly restored gap junctions in the presence of Src and led to dramatic changes in the Cx43 phospho-profile including to increased Y247, Y265, S279/282, S365, and S373 phosphorylation. Lysosomal inhibition, on the other hand, nearly eliminated phosphorylation on Y247 and Y265 and reduced S368 and S373 while increasing S279/282 phosphorylation levels. We present a model of gap junction disassembly where multiple modes of disassembly are regulated by phosphorylation and can have differential effects on cellular signaling. -

Myoplasmic Resting Ca2+ Regulation by Ryanodine Receptors Is
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Georgia State University Georgia State University ScholarWorks @ Georgia State University Chemistry Faculty Publications Department of Chemistry 2014 Myoplasmic resting Ca2+ regulation by ryanodine receptors is under the control of a novel Ca2+- binding region of the receptor Yanyi Chen Georgia State University, [email protected] Shenghui Xue Georgia State University, [email protected] Juan Zou Georgia State University, [email protected] Jose Lopez University of California, Davis Jenny J. Yang Georgia State University, [email protected] See next page for additional authors Follow this and additional works at: http://scholarworks.gsu.edu/chemistry_facpub Part of the Chemistry Commons Recommended Citation Chen, Yanyi; Xue, Shenghui; Zou, Juan; Lopez, Jose; Yang, Jenny J.; and Perez, Claudio, "Myoplasmic resting Ca2+ regulation by ryanodine receptors is under the control of a novel Ca2+-binding region of the receptor" (2014). Chemistry Faculty Publications. Paper 10. http://scholarworks.gsu.edu/chemistry_facpub/10 This Article is brought to you for free and open access by the Department of Chemistry at ScholarWorks @ Georgia State University. It has been accepted for inclusion in Chemistry Faculty Publications by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. Authors Yanyi Chen, Shenghui Xue, Juan Zou, Jose Lopez, Jenny J. Yang, and Claudio Perez This article is available at ScholarWorks @ Georgia State University: http://scholarworks.gsu.edu/chemistry_facpub/10 Biochem. J. (2014) 460, 261–271 (Printed in Great Britain) doi:10.1042/BJ20131553 261 Myoplasmic resting Ca2 + regulation by ryanodine receptors is under the control of a novel Ca2 + -binding region of the receptor Yanyi CHEN*1, Shenghui XUE*1, Juan ZOU*, Jose R. -
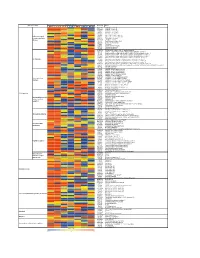
Supporting Figure 2
Normalized value Functional class Genbank Name Naive SC 1h SC 6h SC 24h WM 1h WM 6h WM 24h AB016161 GABA-B receptor 1d AF109405 GABA-B receptor 2a M35077 Dopamine-1A receptor S46131 Dopamine-1A receptor M84009 Dopamine receptor D4 U13368 Adrenergic receptor, alpha 1a G Protein-coupled M60654 Adrenergic receptor, alpha 1d receptors and their M64236 Tachykinin 1 receptor effectors AI229237 Opioid receptor-like Y11433 Pyrimidinergic receptor P2Y4 M64299 Adenosine A1 receptor E00001 Pro-insulin M29014 Insulin receptor precursor U35315 Serotonin receptor 2C S62043 Serotonin receptor 6 AF000368 Scn9a sodium channel, type IX, alpha polypeptide M27158 Kcna5 K+ voltage-gated channel, shaker-related subfamily, member 5 X17621 Kcna6 potassium voltage-gated channel, shaker-related, subfamily, member 6 X16476 Kcnb1 potassium voltage gated channel, Shab-related subfamily, member 1 M77482 Kcnb2 potassium voltage gated channel, Shab-related subfamily, member 2 S64320 Kcnd2 potassium voltage gated channel, Shal-related family, member 2 Ion channels X87635 Kcnj4 potassium inwardly-rectifying channel, subfamily J, member 4 D86039 Kcnj11 potassium inwardly-rectifying channel, subfamily J, member 11 X83581 Kcnj16 potassium inwardly-rectifying channel, subfamily J, member 16 AF073891 Kcnh5 potassium voltage-gated channel, subfamily H (eag-related), member 5 U69882 Kcnn2 potassium intermediate/small conductance calcium-activated channel, subfamily N, member 2 Z36944 Chloride channel 4-2 Z56277 Chloride channel 5 L08493 GABA-A receptor alpha-4 subunit X51992 -

Characterization of Innexin Gene Expression and Functional Roles of Gap-Junctional Communication in Planarian Regeneration
Developmental Biology 287 (2005) 314 – 335 www.elsevier.com/locate/ydbio Characterization of innexin gene expression and functional roles of gap-junctional communication in planarian regeneration Taisaku Nogi, Michael Levin * Department of Cytokine Biology, The Forsyth Institute, 140 The Fenway, Boston, MA 02115, USA Department of Oral and Developmental Biology, Harvard School of Dental Medicine, 140 The Fenway, Boston, MA 02115, USA Received for publication 17 January 2005, revised 20 August 2005, accepted 1 September 2005 Available online 21 October 2005 Abstract Planaria possess remarkable powers of regeneration. After bisection, one blastema regenerates a head, while the other forms a tail. The ability of previously-adjacent cells to adopt radically different fates could be due to long-range signaling allowing determination of position relative to, and the identity of, remaining tissue. However, this process is not understood at the molecular level. Following the hypothesis that gap-junctional communication (GJC) may underlie this signaling, we cloned and characterized the expression of the Innexin gene family during planarian regeneration. Planarian innexins fall into 3 groups according to both sequence and expression. The concordance between expression-based and phylogenetic grouping suggests diversification of 3 ancestral innexin genes into the large family of planarian innexins. Innexin expression was detected throughout the animal, as well as specifically in regeneration blastemas, consistent with a role in long-range signaling relevant to specification of blastema positional identity. Exposure to a GJC-blocking reagent which does not distinguish among gap junctions composed of different Innexin proteins (is not subject to compensation or redundancy) often resulted in bipolar (2-headed) animals. -

Ion Channels 3 1
r r r Cell Signalling Biology Michael J. Berridge Module 3 Ion Channels 3 1 Module 3 Ion Channels Synopsis Ion channels have two main signalling functions: either they can generate second messengers or they can function as effectors by responding to such messengers. Their role in signal generation is mainly centred on the Ca2 + signalling pathway, which has a large number of Ca2+ entry channels and internal Ca2+ release channels, both of which contribute to the generation of Ca2 + signals. Ion channels are also important effectors in that they mediate the action of different intracellular signalling pathways. There are a large number of K+ channels and many of these function in different + aspects of cell signalling. The voltage-dependent K (KV) channels regulate membrane potential and + excitability. The inward rectifier K (Kir) channel family has a number of important groups of channels + + such as the G protein-gated inward rectifier K (GIRK) channels and the ATP-sensitive K (KATP) + + channels. The two-pore domain K (K2P) channels are responsible for the large background K current. Some of the actions of Ca2 + are carried out by Ca2+-sensitive K+ channels and Ca2+-sensitive Cl − channels. The latter are members of a large group of chloride channels and transporters with multiple functions. There is a large family of ATP-binding cassette (ABC) transporters some of which have a signalling role in that they extrude signalling components from the cell. One of the ABC transporters is the cystic − − fibrosis transmembrane conductance regulator (CFTR) that conducts anions (Cl and HCO3 )and contributes to the osmotic gradient for the parallel flow of water in various transporting epithelia. -

Stem Cells and Ion Channels
Stem Cells International Stem Cells and Ion Channels Guest Editors: Stefan Liebau, Alexander Kleger, Michael Levin, and Shan Ping Yu Stem Cells and Ion Channels Stem Cells International Stem Cells and Ion Channels Guest Editors: Stefan Liebau, Alexander Kleger, Michael Levin, and Shan Ping Yu Copyright © 2013 Hindawi Publishing Corporation. All rights reserved. This is a special issue published in “Stem Cells International.” All articles are open access articles distributed under the Creative Com- mons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Editorial Board Nadire N. Ali, UK Joseph Itskovitz-Eldor, Israel Pranela Rameshwar, USA Anthony Atala, USA Pavla Jendelova, Czech Republic Hannele T. Ruohola-Baker, USA Nissim Benvenisty, Israel Arne Jensen, Germany D. S. Sakaguchi, USA Kenneth Boheler, USA Sue Kimber, UK Paul R. Sanberg, USA Dominique Bonnet, UK Mark D. Kirk, USA Paul T. Sharpe, UK B. Bunnell, USA Gary E. Lyons, USA Ashok Shetty, USA Kevin D. Bunting, USA Athanasios Mantalaris, UK Igor Slukvin, USA Richard K. Burt, USA Pilar Martin-Duque, Spain Ann Steele, USA Gerald A. Colvin, USA EvaMezey,USA Alexander Storch, Germany Stephen Dalton, USA Karim Nayernia, UK Marc Turner, UK Leonard M. Eisenberg, USA K. Sue O’Shea, USA Su-Chun Zhang, USA Marina Emborg, USA J. Parent, USA Weian Zhao, USA Josef Fulka, Czech Republic Bruno Peault, USA Joel C. Glover, Norway Stefan Przyborski, UK Contents Stem Cells and Ion Channels, Stefan Liebau, -

An Update on Connexin Gap Junction and Hemichannels in Diabetic Retinopathy
International Journal of Molecular Sciences Review An Update on Connexin Gap Junction and Hemichannels in Diabetic Retinopathy Jorge González-Casanova 1 , Oliver Schmachtenberg 2, Agustín D. Martínez 3, Helmuth A. Sanchez 3, Paloma A. Harcha 3 and Diana Rojas-Gomez 4,* 1 Instituto de Ciencias Biomédicas, Facultad de Ciencias de la Salud, Universidad Autónoma de Chile, Santiago 8910060, Chile; [email protected] 2 Centro Interdisciplinario de Neurociencia de Valparaíso, Instituto de Biología, Facultad de Ciencias, Universidad de Valparaíso, Valparaíso 2360102, Chile; [email protected] 3 Centro Interdisciplinario de Neurociencia de Valparaíso, Instituto de Neurociencia, Facultad de Ciencias, Universidad de Valparaíso, Valparaíso 2360102, Chile; [email protected] (A.D.M.); [email protected] (H.A.S.); [email protected] (P.A.H.) 4 Escuela de Nutrición y Dietética, Facultad de Medicina, Universidad Andres Bello, Santiago 8370146, Chile * Correspondence: [email protected]; Tel.: +56-2-26618559 Abstract: Diabetic retinopathy (DR) is one of the main causes of vision loss in the working age popu- lation. It is characterized by a progressive deterioration of the retinal microvasculature, caused by long-term metabolic alterations inherent to diabetes, leading to a progressive loss of retinal integrity and function. The mammalian retina presents an orderly layered structure that executes initial but complex visual processing and analysis. Gap junction channels (GJC) forming electrical synapses are present in each retinal layer and contribute to the communication between different cell types. Citation: González-Casanova, J.; In addition, connexin hemichannels (HCs) have emerged as relevant players that influence diverse Schmachtenberg, O.; Martínez, A.D.; physiological and pathological processes in the retina. -

Calbindin-D28k and Its Role in Apoptosis: Inhibition of Caspase-3 Activity and Interaction with Pro-Caspase-3 Investigated by In-Situ FRET Microscopy
Dissertation Aus dem Physiologischen Institut Lehrstuhl: Physiologie – Zelluläre Physiologie (Biomedizinisches Zentrum München) der Ludwig-Maximilians-Universität München Vorstand: Prof. Dr. Claudia Veigel Calbindin-D28k and its role in apoptosis: Inhibition of Caspase-3 activity and interaction with Pro-Caspase-3 investigated by in-situ FRET microscopy. Dissertation zum Erwerb des Doktorgrades der Medizin an der Medizinischen Fakultät der Ludwig-Maximilians-Universität zu München vorgelegt von Johannes Lohmeier aus Bong Town, Liberia 2018 Mit Genehmigung der Medizinischen Fakultät der Universität München Berichterstatter: Prof. Dr. Michael Meyer Prof. Dr. Alexander Faussner Mitberichterstatter: Prof. Dr. Nikolaus Plesnila Prof. Dr. Dr. Bernd Sutor Dekan: Prof. Dr. med. dent. Reinhard Hickel Tag der mündlichen Prüfung: 14.06.2018 Eidesstattliche Versicherung Lohmeier, Johannes Name, Vorname Ich erkläre hiermit an Eides statt, dass ich die vorliegende Dissertation mit dem Thema Calbindin-D28k and its role in apoptosis: Inhibition of Caspase-3 activity and interaction with Pro-Caspase-3 investigated by in-situ FRET microscopy. selbständig verfasst, mich außer der angegebenen keiner weiteren Hilfsmittel bedient und alle Erkenntnisse, die aus dem Schrifttum ganz oder annähernd übernommen sind, als solche kenntlich gemacht und nach ihrer Herkunft unter Bezeichnung der Fundstelle einzeln nachgewiesen habe. Ich erkläre des Weiteren, dass die hier vorgelegte Dissertation nicht in gleicher oder in ähnlicher Form bei einer anderen Stelle zur Erlangung