National Survey Zimbabwe Non-Communicable Disease Risk
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Midlands Province Mobile Voter Registration Centres
Midlands Province Mobile Voter Registration Centres Chirumhanzu District Team 1 Ward Centre Dates 18 Mwire primary school 10/06/13-11/06/13 18 Tokwe 4 clinic 12/06/13-13/06/13 18 Chingegomo primary school 14/06/13-15/06/13 16 Chishuku Seondary school 16/06/13-18/06/13 9 Upfumba Secondary school 19/06/13-21/06/13 3 Mutya primary school 22/06/13-24/06/13 2 Gonawapotera secondary school 25/06/13-27/06/13 20 Wildegroove primary school 28/06/13-29/06/13 15 Kushinga primary school 30/06/13-02/07/13 12 Huchu compound 03/07/13-04/-07/13 12 Central estates HQ 5/7/13 20 Mtao/Fair Field compound 6/7/13 12 Chiudza homestead 07/07/13-08/06/13 14 Njerere primary school 9/7/13 Team 2 Ward Centre Dates 22 Hillview Secondary school 10/07/13-12/07/13 17 Lalapanzi Secondary school 13/07/13-15/07/13 16 Makuti homestead 16/06/13-17/06/13 1 Mapiravana Secondary school 18/06/13-19/06/13 9 Siyahukwe Secondary school 20/06/13-23/06/13 4 Chizvinire primary school 24/06/13-25/06/13 21 Mukomberana Seconadry school 26/06/13-29/06/13 20 Union primary school 30/06/13-01/07/13 15 Nyikavanhu primary school 02/07/13-03/07/13 19 Musens primary school 04/07/13-06/07/13 16 Utah primary school 07/7/13-09/07/13 Team 3 Ward Centre Dates 11 Faerdan primary school 10/07/13-11/07/13 11 Chamakanda Secondary school 12/07/13-14/07/13 11 Chamakanda primary school 15/07/13-16/07/13 5 Chizhou Secondary school 17/06/13-16/06/13 3 Chilimanzi primary school 21/06/13-23/06/13 25 Maponda primary school 24/06/13-25/06/13 6 Holy Cross seconadry school 26/06/13-28/06/13 20 New England Secondary -

Government Gazette, 30Th October, 2020 2567
ZIMBABWEAN GOVERNMENT GAZETTE, 30TH OCTOBER, 2020 2567 ZIMBABWEAN GOVERNMENT GAZETTE Published by Authority Vol. XCVIII, No. 116 30th OCTOBER, 2020 Price RTGS$155,00 General Notice 2667 of 2020. MEDICINES AND ALLIED SUBSTANCES CONTROL ACT [CHAPTER 15:03] _________ Refusal to Approve Registration of Veterinary Medicines Following Voluntary Withdrawal _________ IT is hereby notified, in terms of section 34(4) of the Medicines and Allied Substances Control Act [Chapter 15:03], that the Medicines Control Authority has refused to approve the registration of the medicines specified in the Schedule the applicants having withdrawn their application(s) for registration. G. N. MAHLANGU, 30-10-2020. Director-General. SCHEDULE APP NO GENERIC NAME TRADE NAME STRENGTH FORM APPLICANT NAME COOPER ZIMBABWE LTD 29 ANTONY V00739 AMITRAZ TRIATIX TR 50% M/M DIP; TOPICAL AVENUE MSASA HARARE ZIMBABWE COOPER ZIMBABWE LTD 29 ANTONY V00714 AMITRAZ ECTAZ EC DIP 12.5% M/V DIP; TOPICAL AVENUE MSASA HARARE ZIMBABWE NEWCASTLE CLONED VIRUS COOPER ZIMBABWE LTD 29 ANTONY V00782 HIPRAVIR-CLON VACCINE (LIVE) AVENUE MSASA HARARE ZIMBABWE COOPER ZIMBABWE LTD 29 ANTONY V00748 FIPRONIL FIPRONIL 9.7% W/V LIQUID; ORAL AVENUE MSASA HARARE ZIMBABWE NEWCASTLE DISEASE COOPER ZIMBABWE LTD 29 ANTONY V00781 HIPRAVIAR-BPL2 VACCINE (INACTIVATED) AVENUE MSASA HARARE ZIMBABWE INFECTIOUS BURSAL DISEASE HIPRA GUMBORO- COOPER ZIMBABWE LTD 29 ANTONY V00785 VACCINE VIRUS (LIVE) CH/80 AVENUE MSASA HARARE ZIMBABWE INFECTIOUS BURSAL DISEASE HIPRA GUMBORO- COOPER ZIMBABWE LTD 29 ANTONY V00784 VACCINE -

Structure and Condition of Zambezi Valley Dry Forests and Thickets
SSTTRRUUCCTTUURREE AANNDD CCOONNDDIITTIIOONN OOFF ZZAAMMBBEEZZII VVAALLLLEEYY DDRRYY FFOORREESSTTSS AANNDD TTHHIICCKKEETTSS January 2002 Published by The Zambezi Society STRUCTURE AND CONDITION OF ZAMBEZI VALLEY DRY FORESTS AND THICKETS by R.E. Hoare, E.F. Robertson & K.M. Dunham January 2002 Published by The Zambezi Society The Zambezi Society is a non- The Zambezi Society P O Box HG774 governmental membership Highlands agency devoted to the Harare conservation of biodiversity Zimbabwe and wilderness and the Tel: (+263-4) 747002/3/4/5 sustainable use of natural E-mail: [email protected] Website: www.zamsoc.org resources in the Zambezi Basin Zambezi Valley dry forest biodiversity i This report has a series of complex relationships with other work carried out by The Zambezi Society. Firstly, it forms an important part of the research carried out by the Society in connection with the management of elephants and their habitats in the Guruve and Muzarabani districts of Zimbabwe, and the Magoe district of Mozambique. It therefore has implications, not only for natural resource management in these districts, but also for the transboundary management of these resources. Secondly, it relates closely to the work being carried out by the Society and the Biodiversity Foundation for Africa on the identification of community-based mechanisms FOREWORD for the conservation of biodiversity in settled lands. Thirdly, it represents a critically important contribution to the Zambezi Basin Initiative for Biodiversity Conservation (ZBI), a collaboration between the Society, the Biodiversity Foundation for Africa, and Fauna & Flora International. The ZBI is founded on the acquisition and dissemination of good biodiversity information for incorporation into developmental and other planning initiatives. -
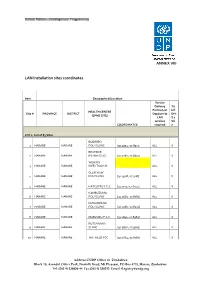
LAN Installation Sites Coordinates
ANNEX VIII LAN Installation sites coordinates Item Geographical/Location Service Delivery Tic Points (List k if HEALTH CENTRE Site # PROVINCE DISTRICT Dept/umits DHI (EPMS SITE) LAN S 2 services Sit COORDINATES required e LOT 1: List of 83 Sites BUDIRIRO 1 HARARE HARARE POLYCLINIC [30.9354,-17.8912] ALL X BEATRICE 2 HARARE HARARE RD.INFECTIO [31.0282,-17.8601] ALL X WILKINS 3 HARARE HARARE INFECTIOUS H ALL X GLEN VIEW 4 HARARE HARARE POLYCLINIC [30.9508,-17.908] ALL X 5 HARARE HARARE HATCLIFFE P.C.C. [31.1075,-17.6974] ALL X KAMBUZUMA 6 HARARE HARARE POLYCLINIC [30.9683,-17.8581] ALL X KUWADZANA 7 HARARE HARARE POLYCLINIC [30.9285,-17.8323] ALL X 8 HARARE HARARE MABVUKU P.C.C. [31.1841,-17.8389] ALL X RUTSANANA 9 HARARE HARARE CLINIC [30.9861,-17.9065] ALL X 10 HARARE HARARE HATFIELD PCC [31.0864,-17.8787] ALL X Address UNDP Office in Zimbabwe Block 10, Arundel Office Park, Norfolk Road, Mt Pleasant, PO Box 4775, Harare, Zimbabwe Tel: (263 4) 338836-44 Fax:(263 4) 338292 Email: [email protected] NEWLANDS 11 HARARE HARARE CLINIC ALL X SEKE SOUTH 12 HARARE CHITUNGWIZA CLINIC [31.0763,-18.0314] ALL X SEKE NORTH 13 HARARE CHITUNGWIZA CLINIC [31.0943,-18.0152] ALL X 14 HARARE CHITUNGWIZA ST.MARYS CLINIC [31.0427,-17.9947] ALL X 15 HARARE CHITUNGWIZA ZENGEZA CLINIC [31.0582,-18.0066] ALL X CHITUNGWIZA CENTRAL 16 HARARE CHITUNGWIZA HOSPITAL [31.0628,-18.0176] ALL X HARARE CENTRAL 17 HARARE HARARE HOSPITAL [31.0128,-17.8609] ALL X PARIRENYATWA CENTRAL 18 HARARE HARARE HOSPITAL [30.0433,-17.8122] ALL X MURAMBINDA [31.65555953980,- 19 MANICALAND -

Midlands Province
School Province District School Name School Address Level Primary Midlands Chirumanzu BARU KUSHINGA PRIMARY BARU KUSHINGA VILLAGE 48 CENTAL ESTATES Primary Midlands Chirumanzu BUSH PARK MUSENA RESETTLEMENT AREA VILLAGE 1 MUSENA Primary Midlands Chirumanzu BUSH PARK 2 VILLAGE 5 WARD 19 CHIRUMANZU Primary Midlands Chirumanzu CAMBRAI ST MATHIAS LALAPANZI TOWNSHIP CHIRUMANZU Primary Midlands Chirumanzu CHAKA NDARUZA VILLAGE HEAD CHAKA Primary Midlands Chirumanzu CHAKASTEAD FENALI VILLAGE NYOMBI SIDING Primary Midlands Chirumanzu CHAMAKANDA TAKAWIRA RESETTLEMENT SCHEME MVUMA Primary Midlands Chirumanzu CHAPWANYA HWATA-HOLYCROSS ROAD RUDUMA VILLAGE Primary Midlands Chirumanzu CHIHOSHO MATARITANO VILLAGE HEADMAN DEBWE Primary Midlands Chirumanzu CHILIMANZI NYONGA VILLAGE CHIEF CHIRUMANZU Primary Midlands Chirumanzu CHIMBINDI CHIMBINDI VILLAGE WARD 5 CHIRUMANZU Primary Midlands Chirumanzu CHINGEGOMO WARD 18 TOKWE 4 VILLAGE 16 CHIRUMANZU Primary Midlands Chirumanzu CHINYUNI CHINYUNI WARD 7 CHUKUCHA VILLAGE Primary Midlands Chirumanzu CHIRAYA (WYLDERGROOVE) MVUMA HARARE ROAD WASR 20 VILLAGE 1 Primary Midlands Chirumanzu CHISHUKU CHISHUKU VILAGE 3 CHIEF CHIRUMANZU Primary Midlands Chirumanzu CHITENDERANO TAKAWIRA RESETTLEMENT AREA WARD 11 Primary Midlands Chirumanzu CHIWESHE PONDIWA VILLAGE MAPIRAVANA Primary Midlands Chirumanzu CHIWODZA CHIWODZA RESETTLEMENT AREA Primary Midlands Chirumanzu CHIWODZA NO 2 VILLAGE 66 CHIWODZA CENTRAL ESTATES Primary Midlands Chirumanzu CHIZVINIRE CHIZVINIRE PRIMARY SCHOOL RAMBANAPASI VILLAGE WARD 4 Primary Midlands -

Mashonaland Central Province : 2013 Harmonised Elections:Presidential Election Results
MASHONALAND CENTRAL PROVINCE : 2013 HARMONISED ELECTIONS:PRESIDENTIAL ELECTION RESULTS Mugabe Robert Mukwazhe Ncube Dabengwa Gabriel (ZANU Munodei Kisinoti Welshman Tsvangirayi Total Votes Ballot Papers Total Valid Votes District Local Authority Constituency Ward No. Polling Station Facility Dumiso (ZAPU) PF) (ZDP) (MDC) Morgan (MDC-T) Rejected Unaccounted for Total Votes Cast Cast Bindura Bindura Municipality Bindura North 1 Bindura Primary Primary School 2 519 0 1 385 6 0 913 907 Bindura Bindura Municipality Bindura North 1 Bindura Hospital Tent 0 525 0 8 521 11 0 1065 1054 2 POLLING STATIONS WARD TOTAL 2 1,044 0 9 906 17 0 1,978 1,961 Bindura Bindura Municipality Bindura North 2 Bindura University New Site 0 183 2 1 86 2 0 274 272 Bindura Bindura Municipality Bindura North 2 Shashi Primary Primary School 0 84 0 2 60 2 0 148 146 Bindura Bindura Municipality Bindura North 2 Zimbabwe Open UniversityTent 2 286 1 4 166 5 0 464 459 3 POLLING STATIONS WARD TOTAL 2 553 3 7 312 9 0 886 877 Bindura Bindura Municipality Bindura North 3 Chipindura High Secondary School 2 80 0 0 81 1 2 164 163 Bindura Bindura Municipality Bindura North 3 DA’s Campus Tent 1 192 0 7 242 2 0 444 442 2 POLLING STATIONS WARD TOTAL 3 272 0 7 323 3 2 608 605 Bindura Bindura Municipality Bindura North 4 Salvation Army Primary School 1 447 0 7 387 12 0 854 842 1 POLLING STATIONS WARD TOTAL 1 447 0 7 387 12 0 854 842 Bindura Bindura Municipality Bindura North 5 Chipadze A Primary School ‘A’ 0 186 2 0 160 4 0 352 348 Bindura Bindura Municipality Bindura North 5 Chipadze B -

MASHONALAND EAST PROVINCE - Basemap
MASHONALAND EAST PROVINCE - Basemap Mashonaland Central Karanda Chimandau Guruve MukosaMukosa Guruve Kamusasa Karanda Marymount Matsvitsi Marymount Mary Mount Locations ShinjeShinje Horseshoe Nyamahobobo Ruyamuro RUSHINGA CentenaryDavid Nelson Nyamatikiti Nyamatikiti Province Capital Nyakapupu M a z o w e CENTENARY Mazowe St. Pius MOUNT DARWIN 2 Chipuriro Mount DarwinZRP NyanzouNyanzou Mt Darwin Chidikamwedzi Town 17 GoromonziNyahuku Tsakare GURUVE Jingamvura MAKONDE Kafura Nyamhondoro Place of Local Importance Bepura 40 Kafura Mugarakamwe Mudindo Nyamanyora Chingamuka Bure Katanya Nyamanyora Bare Chihuri Dindi ARDA Sisi Manga Dindi Goora Mission M u s e n g e z i Nyakasoro KondoKondo Zvomanyanga Goora Wa l t o n Chinehasha Madziwa Chitsungo Mine Silverside Donje Madombwe Mutepatepa Nyamaruro C o w l e y Chistungo Chisvo DenderaDendera Nyamapanda Birkdale Chimukoko Nyamapanda Chindunduma 13 Mukodzongi UMFURUDZI SAFARI AREA Madziwa Chiunye KotwaKotwa 16 Chiunye Shinga Health Facility Nyakudya UZUMBA MARAMBA PFUNGWE Shinga Kotwa Nyakudya Bradley Institute Borera Kapotesa Shopo ChakondaTakawira MvurwiMvurwi Makope Raffingora Jester H y d e Maramba Ayrshire Madziwa Raffingora Mvurwi Farm Health Scheme Nyamaropa MUDZI Kasimbwi Masarakufa Boundaries Rusununguko Madziva Mine Madziwa Vanad R u y a Madziwa Masarakufa Shutu Nyamukoho P e m b i Nzvimbo M u f u r u d z i Madziva Teacher's College Vanad Nzvimbo Chidembo SHAMVA Masenda National Boundary Feock MutawatawaMutawatawa Mudzi Rosa Muswewenhede Chakonda Suswe Mutorashanga Madimutsa Chiwarira -

Back to Index MOBILE REGISTRATION PROGRAMME
Back to index MOBILE REGISTRATION PROGRAMME BIRTHS, DEATHS, NATIONAL AND VOTER REGISTRATION 15 OCTOBER - 13 DECEMBER 2001 as in Herald 12 October 2001 MIDLANDS PROVINCE SHURUGWI DISTRICT Centre Dates 1 Tongogara High School 15/10-17/10 2 Chironde Primary School 18-Oct 3 Hanke Mission School 19/10-20/10 4 Juchuta Secondary School 21/10-22/10 5 Dombwe Primary School 23/10-24/10 6 Svika Secondary Schoo 25/10-27/10 7 Takunda Secondary School 28/10-29/10 8 Chitora Secondary School 30/10-01/11 9 Batanai Primary School 02-Nov 10 Gwanza Primary School 03/11-05/11 11 Tokwe Primary School 06-Nov 12 Gare Secondary School 07/11-09/11 13 Chivakanenyanga Secondary School 10/11-11/11 14 Kushinga Sec School 12/11-13/11 15 Zvamatenga Primary School 14-Nov 16 Gamwa Secondary School 15/11-16/11 17 Gato Secondary School 17/11-18/11 18 Batanai Secondary School 19/11-20/11 19 Rusununguko Sec School 21/11-23/11 20 Donga DDF 24/11-26/11 21 Dombotombo Sec School 27/11-28/11 22 Pakame Secondary School 29/11-30/11 23 Marishongwe Primary School 01-Dec 24 Ruchanyu Primary School 02-Dec 25 Dorset Secondary School 03/12-04/12 26 Adams/Longton Farm 05-Dec 27 Beacon Kop Farm 06-Dec 28 Bethall Primary School 07-Dec 29 Highlands Farmhouse 08-Dec 30 Edwards/Muponda Farmhouse 09-Dec 31 Glentore/Wallclose 10-Dec 32 Chikwingizha Sem/Sec Sch 11-Dec 33 Valley Primary School 12-Dec 34 Zvumwa Secondary School 13-Dec MBERENGWA DISTRICT TEAM ONE Centre Dates 1 New Resettlements 15/10-18/10 2 Murezu Secondary School 19/10-22/10 3 Chizungu Secondary School 23/10-26/10 4 Matobo Secondary -

School Level Province District School Name School Address Secondary
School Level Province District School Name School Address Secondary Midlands Chirumanzu CHAMAKANDA LYNWOOD CENTER TAKAWIRA RESETTLEMENT Secondary Midlands Chirumanzu CHENGWENA RAMBANAPASI VILLAGE, CHIEF HAMA CHIRUMANZU Secondary Midlands Chirumanzu CHISHUKU VILLAGE 2A CHISHUKU RESETLEMENT Secondary Midlands Chirumanzu CHIVONA DENHERE VILLAGE WARD 3 MHENDE CMZ Secondary Midlands Chirumanzu CHIWODZA VILLAGE 38 CHIWODZA RESETTLEMENT MVUMA Secondary Midlands Chirumanzu CHIZHOU WARD 5 MUZEZA VILLAGE, HEADMAN BANGURE , CHIRUMANZU Secondary Midlands Chirumanzu DANNY DANNY SEC Secondary Midlands Chirumanzu DRIEFONTEIN DRIEFONTEIN MISSION FARM Secondary Midlands Chirumanzu GONAWAPOTERA CHAKA BUSINESS CENTRE MVUMA MASVINGO ROAD Secondary Midlands Chirumanzu HILLVIEW HILLVIEW VILLAGE1, LALAPANZI Secondary Midlands Chirumanzu HOLY CROSS HOLY CROSS MISSION WARD 6 CHIRUMANZU Secondary Midlands Chirumanzu LALAPANZI 42KM ALONG GWERU-MVUMA ROAD Secondary Midlands Chirumanzu LEOPOLD TAKAWIRA LEOPOLD TAKAWIRA 2KM ALONG CENTRAL ESTATES ROAD Secondary Midlands Chirumanzu MAPIRAVANA MAPIRAVANA VILLAGE WARD 1CHIRUMANZU Secondary Midlands Chirumanzu MUKOMBERANWA MUWANI VILLAGE HEADMAN MANHOVO Secondary Midlands Chirumanzu MUSENA VILLAGE 8 MUSENA RESETTLEMENT Secondary Midlands Chirumanzu MUSHANDIRAPAMWE RUDHUMA VILLAGE WARD 25 CHIRUMANZU Secondary Midlands Chirumanzu MUTENDERENDE DZORO VILLAGE CHIEF HAMA Secondary Midlands Chirumanzu NEW ENGLAND LOVEDALE FARMSUB-DIVISION 2 MVUMA Secondary Midlands Chirumanzu ORTON'S DRIFT ORTON'S DRIFT FARM Secondary Midlands -

Midlands North Inspection Stations
MIDLANDS NORTH INSPECTION STATIONS GOKWE CENTRAL 1 BHEJANI PRIMARY SCHOOL 2 CHEVECHEVE SECONDARY SCHOOL 3 CHIDAMOYO PRIMARY SCHOOL 4 CHIDOMA PRIMARY SCHOOL 5 CHITAPO BUSINESS CENTRE 6 GANYUNGU PRIMARY SCHOOL 7 GWANYIKA PRIMARY SCHOOL 8 GWENUNGU PRIMARY SCHOOL 9 GWENYA PRIMARY SCHOOL 10 JAHANA PRIMARY SCHOOL 11 KASIKANA PRIMARY SCHOOL 12 KRIMA PRIMARY SCHOOL 13 MALIYAMI PRIMARY SCHOOL 14 MAPU PRIMARY SCHOOL 15 MTANKI PRIMARY SCHOOL 16 MUTANGE C.B. PRIMARY SCHOOL 17 MWEMBESI PRIMARY SCHOOL 18 NDABAMBI PRIMARY SCHOOL 19 NHONGO PRIMARY SCHOOL 20 SATENGWE PRIMARY SCHOOL 21 SENGWA PRIMARY SCHOOL 22 SIMBE PRIMARY SCHOOL 23 ST. CUTHBERTS MASORO PRIMARY SCHOOL 24 TONGWE SECONDARY SCHOOL 25 CHIZIYA COMMUNITY HALL 26 GABABE PRIMARY SCHOOL 27 RAJI MINISTRY OF TRANSPORT 28 GWEHAVA PRIMARY SCHOOL GOKWE EAST 1 BATSIRAI PRIMARY SCHOOL 2 CHAMINUKA PRIMARY SCHOOL 3 CHINYENYETU PRIMARY SCHOOL 4 CHIODZA PRIMARY SCHOOL 5 COPPER QUEEN BUSINESS CENTRE 6 DENDA PRIMARY SCHOOL 7 DEWE PRIMARY SCHOOL 8 DINDIMUTIWI PRIMARY SCHOOL 9 GANDAVACHE PRIMARY SCHOOL 10 GOREDEMA PRIMARY SCHOOL 11 GWEBO PRIMARY SCHOOL 12 HWADZE PRIMARY SCHOOL 13 KAHOBO PRIMARY SCHOOL 14 KAMWA PRIMARY SCHOOL 15 KAMWAMBE PRIMARY SCHOOL 16 KASONDE PRIMARY SCHOOL 17 KUEDZA PRIMARY SCHOOL 18 KWAEDZA PRIMARY SCHOOL 19 MADZIDRIRE PRIMARY SCHOOL 20 MASOSONI PRIMARY SCHOOL 21 MHUMHA PRIMARY SCHOOL 22 MSADZI PRIMARY SCHOOL 23 MUDONDO PRIMARY SCHOOL 24 MUPAWA PRIMARY SCHOOL 25 MUSOROWENZOU PRIMARY SCHOOL 26 MUTEHWE PRIMARY SCHOOL 27 MUTUKANYI PRIMARY SCHOOL 28 NORAH PRIMARY SCHOOL 29 NYAMAZENGWE PRIMARY -

Political Violence Report November 2001
ZIMBABWE HUMAN RIGHTS NGO FORUM POLITICAL VIOLENCE REPORT NOVEMBER 2001 December 2001 A report by the Zimbabwe Human Rights NGO Forum Summary Attacks on farm workers residing on invaded farms, ongoing since the end of February 2000, were reported to the Human Rights Forum in November. Since January this year, more than 70 000 farm workers have been displaced and countless others have been beaten and tortured whilst others lost their homes in arson attacks. Once again the ZRP was been found wanting in its protection and reaction to violations concerning farm workers and MDC supporters. In one recorded case, policemen in Kadoma arrived at the local Zanu-PF offices and witnessed the torture of two MDC members but left without rendering assistance. However 14 MDC members were arrested for the murder of Bulawayo war veteran’s chairman, Cain Nkala. In Harare members of the ZRP unlawfully arrested and tortured two MDC officials from Zengeza, accusing them of having had some involvement in Cain Nkala’s murder, despite their being in Harare at the time. The two were released without being charged having been interrogated about the operations of their party. Six deaths were recorded in November. A list of all deaths that have been reported since January 2001 is given at the end of the report. November 2001 Totals 2 Cumulative Totals January-November 2001 Sources: Amani Trust medical assessments, HR Forum legal statements, CFU reports and newspaper reports. Notes to Tables: The following categories have been changed and/or expanded due to the nature and the volume of crimes. -

Secondary School Children's Experiences of Bereavement: Implications for School Counselling in Harare Metropolitan Province By
SECONDARY SCHOOL CHILDREN’S EXPERIENCES OF BEREAVEMENT: IMPLICATIONS FOR SCHOOL COUNSELLING IN HARARE METROPOLITAN PROVINCE BY Jenny Shumba A thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy In the Faculty of Education at the UNIVERSITY OF FORT HARE Promoter: Prof. George Moyo 2011 DECLARATION This thesis is my original work. The work contained in it has not been previously submitted to meet requirements for an award at this or any other higher education institution. To the best of my knowledge and belief, this thesis contains no material previously published or written by another person except where due reference is made. Jenny Shumba ……………………………………………………. Date: ……………………………………………………… i ABSTRACT Death and bereavement are prevalent in Zimbabwe due to HIV/AIDS and other illnesses. It is estimated that a large population of school going learners have lost one or both parents and have become orphans. The aim of the study was two-fold: to understand the bereavement experiences of orphaned learners and to examine how such experiences can inform school counselling services. A multiple case study involving 13 school children and four school counsellors from two secondary schools in Harare Metropolitan Region was conducted. Each of the 17 participants was viewed as a bounded case due to his or her individual unique experiences. An interpretive phenomenological approach was employed to collect and analyse the data. All the cases were purposively selected as they were bearers of crucial information on bereavement experiences and bereavement counselling. The study established nine (9) key findings. First, it was found that the type of attachments and support systems the child had were contributory to the way the child experienced bereavement.