<I>Bacillus Thuringiensis</I>
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ARTHROPOD COMMUNITIES and PASSERINE DIET: EFFECTS of SHRUB EXPANSION in WESTERN ALASKA by Molly Tankersley Mcdermott, B.A./B.S
Arthropod communities and passerine diet: effects of shrub expansion in Western Alaska Item Type Thesis Authors McDermott, Molly Tankersley Download date 26/09/2021 06:13:39 Link to Item http://hdl.handle.net/11122/7893 ARTHROPOD COMMUNITIES AND PASSERINE DIET: EFFECTS OF SHRUB EXPANSION IN WESTERN ALASKA By Molly Tankersley McDermott, B.A./B.S. A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Biological Sciences University of Alaska Fairbanks August 2017 APPROVED: Pat Doak, Committee Chair Greg Breed, Committee Member Colleen Handel, Committee Member Christa Mulder, Committee Member Kris Hundertmark, Chair Department o f Biology and Wildlife Paul Layer, Dean College o f Natural Science and Mathematics Michael Castellini, Dean of the Graduate School ABSTRACT Across the Arctic, taller woody shrubs, particularly willow (Salix spp.), birch (Betula spp.), and alder (Alnus spp.), have been expanding rapidly onto tundra. Changes in vegetation structure can alter the physical habitat structure, thermal environment, and food available to arthropods, which play an important role in the structure and functioning of Arctic ecosystems. Not only do they provide key ecosystem services such as pollination and nutrient cycling, they are an essential food source for migratory birds. In this study I examined the relationships between the abundance, diversity, and community composition of arthropods and the height and cover of several shrub species across a tundra-shrub gradient in northwestern Alaska. To characterize nestling diet of common passerines that occupy this gradient, I used next-generation sequencing of fecal matter. Willow cover was strongly and consistently associated with abundance and biomass of arthropods and significant shifts in arthropod community composition and diversity. -

Oak Woodland Litter Spiders James Steffen Chicago Botanic Garden
Oak Woodland Litter Spiders James Steffen Chicago Botanic Garden George Retseck Objectives • Learn about Spiders as Animals • Learn to recognize common spiders to family • Learn about spider ecology • Learn to Collect and Preserve Spiders Kingdom - Animalia Phylum - Arthropoda Subphyla - Mandibulata Chelicerata Class - Arachnida Orders - Acari Opiliones Pseudoscorpiones Araneae Spiders Arachnids of Illinois • Order Acari: Mites and Ticks • Order Opiliones: Harvestmen • Order Pseudoscorpiones: Pseudoscorpions • Order Araneae: Spiders! Acari - Soil Mites Characteriscs of Spiders • Usually four pairs of simple eyes although some species may have less • Six pair of appendages: one pair of fangs (instead of mandibles), one pair of pedipalps, and four pair of walking legs • Spinnerets at the end of the abdomen, which are used for spinning silk threads for a variety of purposes, such as the construction of webs, snares, and retreats in which to live or to wrap prey • 1 pair of sensory palps (often much larger in males) between the first pair of legs and the chelicerae used for sperm transfer, prey manipulation, and detection of smells and vibrations • 1 to 2 pairs of book-lungs on the underside of abdomen • Primitively, 2 body regions: Cephalothorax, Abdomen Spider Life Cycle • Eggs in batches (egg sacs) • Hatch inside the egg sac • molt to spiderlings which leave from the egg sac • grows during several more molts (instars) • at final molt, becomes adult – Some long-lived mygalomorphs (tarantulas) molt after adulthood Phenology • Most temperate -

Spider Bites
Infectious Disease Epidemiology Section Office of Public Health, Louisiana Dept of Health & Hospitals 800-256-2748 (24 hr number) www.infectiousdisease.dhh.louisiana.gov SPIDER BITES Revised 6/13/2007 Epidemiology There are over 3,000 species of spiders native to the United States. Due to fragility or inadequate length of fangs, only a limited number of species are capable of inflicting noticeable wounds on human beings, although several small species of spiders are able to bite humans, but with little or no demonstrable effect. The final determination of etiology of 80% of suspected spider bites in the U.S. is, in fact, an alternate diagnosis. Therefore the perceived risk of spider bites far exceeds actual risk. Tick bites, chemical burns, lesions from poison ivy or oak, cutaneous anthrax, diabetic ulcer, erythema migrans from Lyme disease, erythema from Rocky Mountain Spotted Fever, sporotrichosis, Staphylococcus infections, Stephens Johnson syndrome, syphilitic chancre, thromboembolic effects of Leishmaniasis, toxic epidermal necrolyis, shingles, early chicken pox lesions, bites from other arthropods and idiopathic dermal necrosis have all been misdiagnosed as spider bites. Almost all bites from spiders are inflicted by the spider in self defense, when a human inadvertently upsets or invades the spider’s space. Of spiders in the United States capable of biting, only a few are considered dangerous to human beings. Bites from the following species of spiders can result in serious sequelae: Louisiana Office of Public Health – Infectious Disease Epidemiology Section Page 1 of 14 The Brown Recluse: Loxosceles reclusa Photo Courtesy of the Texas Department of State Health Services The most common species associated with medically important spider bites: • Physical characteristics o Length: Approximately 1 inch o Appearance: A violin shaped mark can be visualized on the dorsum (top). -

2017 AAS Abstracts
2017 AAS Abstracts The American Arachnological Society 41st Annual Meeting July 24-28, 2017 Quéretaro, Juriquilla Fernando Álvarez Padilla Meeting Abstracts ( * denotes participation in student competition) Abstracts of keynote speakers are listed first in order of presentation, followed by other abstracts in alphabetical order by first author. Underlined indicates presenting author, *indicates presentation in student competition. Only students with an * are in the competition. MAPPING THE VARIATION IN SPIDER BODY COLOURATION FROM AN INSECT PERSPECTIVE Ajuria-Ibarra, H. 1 Tapia-McClung, H. 2 & D. Rao 1 1. INBIOTECA, Universidad Veracruzana, Xalapa, Veracruz, México. 2. Laboratorio Nacional de Informática Avanzada, A.C., Xalapa, Veracruz, México. Colour variation is frequently observed in orb web spiders. Such variation can impact fitness by affecting the way spiders are perceived by relevant observers such as prey (i.e. by resembling flower signals as visual lures) and predators (i.e. by disrupting search image formation). Verrucosa arenata is an orb-weaving spider that presents colour variation in a conspicuous triangular pattern on the dorsal part of the abdomen. This pattern has predominantly white or yellow colouration, but also reflects light in the UV part of the spectrum. We quantified colour variation in V. arenata from images obtained using a full spectrum digital camera. We obtained cone catch quanta and calculated chromatic and achromatic contrasts for the visual systems of Drosophila melanogaster and Apis mellifera. Cluster analyses of the colours of the triangular patch resulted in the formation of six and three statistically different groups in the colour space of D. melanogaster and A. mellifera, respectively. Thus, no continuous colour variation was found. -

The Dynamics of Endosymbiotic Infections in a Linyphiid Spider
University of Kentucky UKnowledge Theses and Dissertations--Entomology Entomology 2020 A Tangled Web: The Dynamics of Endosymbiotic Infections in a Linyphiid Spider Laura Cecilia Rosenwald University of Kentucky, [email protected] Author ORCID Identifier: https://orcid.org/0000-0001-7846-644X Digital Object Identifier: https://doi.org/10.13023/etd.2020.424 Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Rosenwald, Laura Cecilia, "A Tangled Web: The Dynamics of Endosymbiotic Infections in a Linyphiid Spider" (2020). Theses and Dissertations--Entomology. 58. https://uknowledge.uky.edu/entomology_etds/58 This Master's Thesis is brought to you for free and open access by the Entomology at UKnowledge. It has been accepted for inclusion in Theses and Dissertations--Entomology by an authorized administrator of UKnowledge. For more information, please contact [email protected]. STUDENT AGREEMENT: I represent that my thesis or dissertation and abstract are my original work. Proper attribution has been given to all outside sources. I understand that I am solely responsible for obtaining any needed copyright permissions. I have obtained needed written permission statement(s) from the owner(s) of each third-party copyrighted matter to be included in my work, allowing electronic distribution (if such use is not permitted by the fair use doctrine) which will be submitted to UKnowledge as Additional File. I hereby grant to The University of Kentucky and its agents the irrevocable, non-exclusive, and royalty-free license to archive and make accessible my work in whole or in part in all forms of media, now or hereafter known. -

Development of the Cursorial Spider, Cheiracanthium Inclusum (Araneae: Miturgidae), on Eggs of Helicoverpa Zea (Lepidoptera: Noctuidae)1
Development of the Cursorial Spider, Cheiracanthium inclusum (Araneae: Miturgidae), on Eggs of Helicoverpa zea (Lepidoptera: Noctuidae)1 R. S. Pfannenstiel2 Beneficial Insects Research Unit, USDA-ARS, Weslaco, Texas 78596 USA J. Entomol. Sci. 43(4): 418422 (October 2008) Abstract Development of the cursorial spider, Cheiracanthium inclusum (Hentz) (Araneae: Miturgidae), from emergence to maturity on a diet of eggs of the lepidopteran pest Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) was characterized. Cheiracanthium inclusum developed to adulthood with no mortality while feeding on a diet solely of H. zea eggs and water. The number of instars to adulthood varied from 4-5 for males and from 4-6 for females, although most males (84.6%) and females (66.7%) required 5 instars. Males and females took a similar time to become adults (54.2 ± 4.0 and 53.9 ± 2.0 days, respectively). Egg consumption was similar between males and females for the first 4 instars, but differed for the 51 instar and for the total number of eggs consumed to reach adulthood (651.0 ± 40.3 and 866.5 ± 51.4 eggs for males and females, respectively). Individual consumption rates suggest the potential for high impact of C. inclusum individuals on pest populations. Development was faster and survival greater than in previous studies of C. inc/usum development. Key Words spider development, egg predation Spiders have been observed feeding on lepidopteran eggs in several crops (re- viewed by Nyffeler et al. 1990), but only recently has the frequency of these obser- vations (Pfannenstiel and Yeargan 2002, Pfartnenstiel 2005, 2008) suggested that lepidopteran eggs may be a common prey item for some families of cursorial spiders. -

David Penney
ARTÍCULO: NEW EXTANT AND FOSSIL DOMINICAN REPUBLIC SPIDER RECORDS, WITH TWO NEW SYNONYMIES AND COMMENTS ON TAPHONOMIC BIAS OF AMBER PRESERVATION David Penney Abstract: A collection of 23 identifiable extant spider species from the Dominican Republic revealed eight (= 35%) new species records for the country and five (= 22%) for the island of Hispaniola. The collection includes the first record of the family Prodidomidae from Hispaniola. Phantyna guanica (Gertsch, 1946) is identified as a junior synonym of Emblyna altamira (Gertsch & Davis, 1942) (Dictynidae) and Ceraticelus solitarius Bryant, 1948 is identified as a junior synonym of C. paludigenus Crosby & Bishop, 1925 (Linyphiidae). Such a large proportion of new records in such a small sample demonstrates that the extant spider fauna of the Dominican Republic is poorly known ARTÍCULO: and is worthy of further investigation, particularly in light of its potential for quantifying New extant and fossil Dominican bias associated with the amber-preserved fauna. New records of fossil spider species Republic spider records, with two preserved in Miocene amber are provided. The taphonomic bias towards a significantly new synonymies and comments higher number of male compared to female spiders as inclusions in Dominican Republic on taphonomic bias of amber amber is a genuine phenomenon. preservation Key words: Arachnida, Araneae, Dictynidae, Linyphiidae, Miocene, palaeontology, taphonomy, taxonomy, Hispaniola. David Penney Taxonomy: Department of Earth Sciences Emblyna altamira (Gertsch & Davis, -

Effects of Climate Change on Arctic Arthropod Assemblages and Distribution Phd Thesis
Effects of climate change on Arctic arthropod assemblages and distribution PhD thesis Rikke Reisner Hansen Academic advisors: Main supervisor Toke Thomas Høye and co-supervisor Signe Normand Submitted 29/08/2016 Data sheet Title: Effects of climate change on Arctic arthropod assemblages and distribution Author University: Aarhus University Publisher: Aarhus University – Denmark URL: www.au.dk Supervisors: Assessment committee: Arctic arthropods, climate change, community composition, distribution, diversity, life history traits, monitoring, species richness, spatial variation, temporal variation Date of publication: August 2016 Please cite as: Hansen, R. R. (2016) Effects of climate change on Arctic arthropod assemblages and distribution. PhD thesis, Aarhus University, Denmark, 144 pp. Keywords: Number of pages: 144 PREFACE………………………………………………………………………………………..5 LIST OF PAPERS……………………………………………………………………………….6 ACKNOWLEDGEMENTS……………………………………………………………………...7 SUMMARY……………………………………………………………………………………...8 RESUMÉ (Danish summary)…………………………………………………………………....9 SYNOPSIS……………………………………………………………………………………....10 Introduction……………………………………………………………………………………...10 Study sites and approaches……………………………………………………………………...11 Arctic arthropod community composition…………………………………………………….....13 Potential climate change effects on arthropod composition…………………………………….15 Arctic arthropod responses to climate change…………………………………………………..16 Future recommendations and perspectives……………………………………………………...20 References………………………………………………………………………………………..21 PAPER I: High spatial -

A Protocol for Online Documentation of Spider Biodiversity Inventories Applied to a Mexican Tropical Wet Forest (Araneae, Araneomorphae)
Zootaxa 4722 (3): 241–269 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2020 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4722.3.2 http://zoobank.org/urn:lsid:zoobank.org:pub:6AC6E70B-6E6A-4D46-9C8A-2260B929E471 A protocol for online documentation of spider biodiversity inventories applied to a Mexican tropical wet forest (Araneae, Araneomorphae) FERNANDO ÁLVAREZ-PADILLA1, 2, M. ANTONIO GALÁN-SÁNCHEZ1 & F. JAVIER SALGUEIRO- SEPÚLVEDA1 1Laboratorio de Aracnología, Facultad de Ciencias, Departamento de Biología Comparada, Universidad Nacional Autónoma de México, Circuito Exterior s/n, Colonia Copilco el Bajo. C. P. 04510. Del. Coyoacán, Ciudad de México, México. E-mail: [email protected] 2Corresponding author Abstract Spider community inventories have relatively well-established standardized collecting protocols. Such protocols set rules for the orderly acquisition of samples to estimate community parameters and to establish comparisons between areas. These methods have been tested worldwide, providing useful data for inventory planning and optimal sampling allocation efforts. The taxonomic counterpart of biodiversity inventories has received considerably less attention. Species lists and their relative abundances are the only link between the community parameters resulting from a biotic inventory and the biology of the species that live there. However, this connection is lost or speculative at best for species only partially identified (e. g., to genus but not to species). This link is particularly important for diverse tropical regions were many taxa are undescribed or little known such as spiders. One approach to this problem has been the development of biodiversity inventory websites that document the morphology of the species with digital images organized as standard views. -

World Spider Catalog (Accessed 4 January 2020) Family: Thomisidae Sundevall, 1833
World Spider Catalog (accessed 4 January 2020) Family: Thomisidae Sundevall, 1833 Gen. Bassaniana Strand, 1928 Bassaniana floridana (Banks, 1896) AL, AR, FL, GA, LA, MD, MS, NJ, OH, SC, TX, VA Bassaniana utahensis (Gertsch, 1932) AB, BC, LB, MB, NB, NF, NS, NT, NU, ON, PQ, SK; AK, AZ, CA, CO, FL, ID, IL, MA, ME, MI, MN, MS, MT, ND, NH, NM, NV, NY, OH, OR, PA, SD, TX, UT, VT, WA, WI Bassaniana versicolor (Keyserling, 1880) ON; AL, AR, AZ, CT, FL, IA, IL, IN, KS, KY, LA, MA, MD, MI, MO, MS, NC, NE, NM, NY, OH, OR, PA, RI, TN, TX, VA, WI, WV Gen. Bucranium O. Pickard-Cambridge, 1881 Bucranium sp. undescribed TX Gen. Coriarachne Thorell, 1870 Coriarachne brunneipes Banks, 1893 AB, BC, MB, NT, ON, PQ, SK; AK, AZ, CA, CO, ID, NV, OR, WA, WY Gen. Diaea Thorell, 1869 Diaea livens Simon, 1876 CA Diaea seminola Gertsch, 1939 FL Gen. Mecaphesa Simon, 1900 Mecaphesa aikoae (Schick, 1965) CA Mecaphesa asperata (Hentz, 1847) AB, BC, MB, ON, PQ, SK; AL, AR, CA, CO, CT, DC, FL, GA, ID, IL, IN, KS, KY, LA, MA, MD, MI, MN, MO, NC, NE, NH, NJ, NM, NY, OH, OK, PA, RI, TN, TX, UT, VA, WI Mecaphesa californica (Banks, 1896) CA, CO, TX, UT Mecaphesa carletonica (Dondale & Redner, 1976) ON, PC; IN, TX Mecaphesa celer (Hentz, 1847) AB, BC, SK; AL, AZ, CA, CO, FL, GA, ID, IL, IN, KS, LA, MA, MI, MN, MO, MS, NC, NE, NM, NV, NY, OH, OK, OR, TX, UT, VA, WA, WY Mecaphesa coloradensis (Gertsch, 1933) AZ, CO, TX, UT Mecaphesa deserti (Schick, 1965) CA Mecaphesa devia (Gertsch, 1939) CA Mecaphesa dubia (Keyserling, 1880) AZ, CA, FL, KS, LA, MS, OK, TX Mecaphesa gabrielensis (Schick, 1965) CA Mecaphesa importuna (Keyserling, 1881) CA Mecaphesa importuna belkini (Schick, 1965) CA Mecaphesa lepida (Thorell, 1877) CA, UT Mecaphesa lowriei (Schick, 1970) CA Mecaphesa quercina (Schick, 1965) CA Mecaphesa rothi (Schick, 1965) CA Mecaphesa schlingeri (Schick, 1965) CA Mecaphesa sierrensis (Schick, 1965) BC Mecaphesa verityi (Schick, 1965) CA Gen. -

Overview of the Anyphaenids (Araneae, Anyphaeninae, Anyphaenidae) Spider Fauna from the Chocó Forest of Ecuador, with the Description of Thirteen New Species
European Journal of Taxonomy 255: 1–50 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2016.255 www.europeanjournaloftaxonomy.eu 2016 · Dupérré N. & Tapia E. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:0E8DA4DC-FF4C-436E-94FB-CB89F6416C6E Overview of the Anyphaenids (Araneae, Anyphaeninae, Anyphaenidae) spider fauna from the Chocó forest of Ecuador, with the description of thirteen new species Nadine DUPÉRRÉ 1,* & Elicio TAPIA 2 1 Research Associate, Fundación OTONGA, Calle Rither y Bolivia, Quito, Ecuador, and Research Associate, American Museum of Natural History, New York, NY, U.S.A. 2 Researcher, Centro Jambatu de Investigación y Conservación de Anfibios, Geovanny Farina 566, San Rafael, Ecuador. * Corresponding author: [email protected] 2 Email: [email protected] 1 urn:lsid:zoobank.org:author:F15E1FF2-2DF5-479A-AD10-8076CE96E911 2 urn:lsid:zoobank.org:author:E842405B-5E5B-43AB-8BCD-586657AD5CFC Abstract. The spider diversity of the family Anyphaenidae in premontane, low evergreen montane and cloud forest from the Chocó region of Ecuador is examined. A total of 287 adult specimens were collected and 19 morphospecies were identified based on male specimens. Thirteen new species are described and one new genus is proposed. Five new species are described in the genus Katissa Brescovit, 1997: Katissa kurusiki sp. nov., K. puyu sp. nov., K. tamya sp. nov., K. yaya sp. nov. and K. guyasamini sp. nov. The new genus Shuyushka gen. nov. is proposed and includes three species: Shuyushka achachay gen. et sp. nov., S. moscai gen. et sp. nov. and S. -
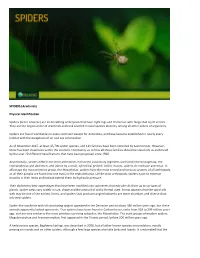
Arachnids) Physical Identification Spiders (Order Araneae
SPIDERS (Arachnids) Physical Identification Spiders (order Araneae) are air-breathing arthropods that have eight legs and chelicerae with fangs that inject venom. They are the largest order of arachnids and rank seventh in total species diversity among all other orders of organisms. Spiders are found worldwide on every continent except for Antarctica, and have become established in nearly every habitat with the exceptions of air and sea colonization. As of November 2015, at least 45,700 spider species, and 113 families have been recorded by taxonomists. However, there has been dissension within the scientific community as to how all these families should be classified, as evidenced by the over 20 different classifications that have been proposed since 1900. Anatomically, spiders differ from other arthropods in that the usual body segments are fused into two tagmata, the cephalothorax and abdomen, and joined by a small, cylindrical pedicel. Unlike insects, spiders do not have antennae. In all except the most primitive group, the Mesothelae, spiders have the most centralized nervous systems of all arthropods, as all their ganglia are fused into one mass in the cephalothorax. Unlike most arthropods, spiders have no extensor muscles in their limbs and instead extend them by hydraulic pressure. Their abdomens bear appendages that have been modified into spinnerets that extrude silk from up to six types of glands. Spider webs vary widely in size, shape and the amount of sticky thread used. It now appears that the spiral orb web may be one of the earliest forms, and spiders that produce tangled cobwebs are more abundant and diverse than orb-web spiders.