Necklace-Style Radio-Transmitters Are Associated with Changes in Display Vocalizations of Male Greater Sage-Grouse Author(S): Marcella R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Necklace-Style Radio-Transmitters Are Associated with Changes in Display Vocalizations of Male Greater Sage-Grouse Authors: Marcella R
Necklace-style radio-transmitters are associated with changes in display vocalizations of male greater sage-grouse Authors: Marcella R. Fremgen, Daniel Gibson, Rebecca L. Ehrlich, Alan H. Krakauer, Jennifer S. Forbey, et. al. Source: Wildlife Biology, 2017(SP1) Published By: Nordic Board for Wildlife Research URL: https://doi.org/10.2981/wlb.00236 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Downloaded From: https://bioone.org/journals/Wildlife-Biology on 2/11/2019 Terms of Use: https://bioone.org/terms-of-use Wildlife Biology 2017: wlb.00236 doi: 10.2981/wlb.00236 © 2016 The Authors. This is an Open Access article Subject Editor: Olafur Nielsen. Editor-in-Chief: Ilse Storch. Accepted 19 July 2016 Necklace-style radio-transmitters are associated with changes in display vocalizations of male greater sage-grouse Marcella R. Fremgen, Daniel Gibson, Rebecca L. Ehrlich, Alan H. -

Birds of Bharatpur – Check List
BIRDS OF BHARATPUR – CHECK LIST Family PHASIANIDAE: Pheasants, Partridges, Quail Check List BLACK FRANCOLIN GREY FRANCOLIN COMMON QUAIL RAIN QUAIL JUNGLE BUSH QUAIL YELLOW-LEGGED BUTTON QUAIL BARRED BUTTON QUAIL PAINTED SPURFOWL INDIAN PEAFOWL Family ANATIDAE: Ducks, Geese, Swans GREATER WHITE-FRONTED GOOSE GREYLAG GOOSE BAR-HEADED GOOSE LWSSER WHISTLING-DUCK RUDDY SHELDUCK COMMON SHELDUCK COMB DUCK COTTON PYGMY GOOSE MARBLED DUCK GADWALL FALCATED DUCK EURASIAN WIGEON MALLARD SPOT-BILLED DUCK COMMON TEAL GARGANEY NORTHERN PINTAIL NORTHERN SHOVELER RED-CRESTED POCHARD COMMON POCHARD FERRUGINOUS POCHARD TUFTED DUCK BAIKAL TEAL GREATER SCAUP BAER’S POCHARD Family PICIDAE: Woodpeckers EURASIAN WRYNECK BROWN-CAPPED PYGMY WOODPECKER YELLOW-CROWNED WOODPECKER BLACK-RUMPED FLAMBACK Family CAPITONIDAE: Barbets BROWN-HEADED BARBET COPPERSMITH BARBET Family UPUPIDAE: Hoopoes COMMON HOOPOE Family BUCEROTIDAE: Hornbills INDAIN GREY HORNBILL Family CORACIIDAE: Rollers or Blue Jays EUROPEAN ROLLER INDIAN ROLLER Family ALCEDINIDAE: Kingfisher COMMON KINGFISHER STORK-BILLED KINGFISHER WHITE-THROATED KINGFISHER BLACK-CAPPED KINGFISHER PIED KINGFISHER Family MEROPIDAE: Bee-eaters GREEN BEE-EATER BLUE-CHEEKED BEE-EATER BLUE-TAILED BEE-EATER Family CUCULIDAE: Cuckoos, Crow-pheasants PIED CUCKOO CHESTNUT-WINGED CUCKOO COMMON HAWK CUCKOO INDIAN CUCKOO EURASIAN CUCKOO GREY-BELLIED CUCKOO PLAINTIVE CUCKOO DRONGO CUCKOO ASIAN KOEL SIRKEER MALKOHA GREATER COUCAL LESSER COUCAL Family PSITTACIDAS: Parrots ROSE-RINGED PARAKEET PLUM-HEADED PARKEET Family APODIDAE: -

Supplementary Material
Pterocles alchata (Pin-tailed Sandgrouse) European Red List of Birds Supplementary Material The European Union (EU27) Red List assessments were based principally on the official data reported by EU Member States to the European Commission under Article 12 of the Birds Directive in 2013-14. For the European Red List assessments, similar data were sourced from BirdLife Partners and other collaborating experts in other European countries and territories. For more information, see BirdLife International (2015). Contents Reported national population sizes and trends p. 2 Trend maps of reported national population data p. 3 Sources of reported national population data p. 5 Species factsheet bibliography p. 6 Recommended citation BirdLife International (2015) European Red List of Birds. Luxembourg: Office for Official Publications of the European Communities. Further information http://www.birdlife.org/datazone/info/euroredlist http://www.birdlife.org/europe-and-central-asia/european-red-list-birds-0 http://www.iucnredlist.org/initiatives/europe http://ec.europa.eu/environment/nature/conservation/species/redlist/ Data requests and feedback To request access to these data in electronic format, provide new information, correct any errors or provide feedback, please email [email protected]. THE IUCN RED LIST OF THREATENED SPECIES™ BirdLife International (2015) European Red List of Birds Pterocles alchata (Pin-tailed Sandgrouse) Table 1. Reported national breeding population size and trends in Europe1. Country (or Population estimate Short-term -

Transport of Water by Adult Sandgrouse to Their Young Tom J
THE CONDOR VOLUME69 JULY-AUGUST,1967 NUMBER4 TRANSPORT OF WATER BY ADULT SANDGROUSE TO THEIR YOUNG TOM J. CADE and GORDONL. MACLEAN In 1896 the English aviculturist Meade-Waldo published an astonishing and seemingly incredible account of how the males of sandgrouse that he successfully bred in captivity carried water to their young in their breast feathers. To quote from his original report: As soon as the young were out of the nest (when twelve hours old) a very curious habit developed itself in the male. He would rub his breast violently up and down on the ground, a motion quite distinct from dusting, and when all awry he would get into his drinking water and saturate the feathers of the under parts. When soaked he would go through the motions of flying away, nodding his head, etc. Then, remembering his family were close by, would run up to the hen, make a demonstration, when the young would run out, get under him, and suckthe water from his breast. This is no doubt the way that water is conveyed to the young when far out on waterless plains. The young . are very independent, eating hard seed and weeds from the first, and roosting independently of their parents at ten days old (Meade-Waldo, 1896). See also Meade- Waldo (1921). Despite the fact that .Meade-Waldo (1897 ; 1921) observed 61 broods from three different species of sandgrouse hatched in his aviaries between 189.5 and l915, and soon received confirmation from another breeder for two species (St. Quintin, 1905), and despite the fact that field naturalists and native hunters have frequently observed wild male sandgrouse wetting their breast feathers at water holes in the way described (Meade-Waldo, 1906; Buxton, 1923; Heim de Balsac, 1936; Hoesch, 1955), the idea that the young do receive water in this exceptional way has met with a great deal of scepticism (Archer and Godman, 1937; Meinertzhagen, 1954, 1964; Hiie and Etchkcopar, 1957; Schmidt-Nielsen, 1964). -

The Water-Holding Mechanism of Sandgrouse Feathers by A
'. Exp. BM. (1972), 56, 195-200 ith 2 text-figures Printed in Great Britain THE WATER-HOLDING MECHANISM OF SANDGROUSE FEATHERS BY A. M. RIJKE Department of Materials Science, University of Virginia, Charlottesville, Virginia 22901 {Received 15 June 1971) INTRODUCTION The detailed studies of Cade & Maclean (1967) on the mechanism of water transport by the sandgrouse Pterocles have contributed important evidence to the long-standing controversy regarding the manner in which the young obtain their drinking water. The field observations of these authors have shown conclusively that the male of the species soaks his abdominal feathers by squatting down in water-holes, usually during the early hours. He then speeds off to the nesting site where the young gather around his exposed abdomen and obtain their water by stripping his belly feathers with their beaks. In addition to this behaviour it has been noted that the abdominal feathers of the male, and to a lesser extent those of the female, show a number of structural charac- teristics which render the uptake of water particularly effective. The distal fifths of these feathers are very similar to the feathers of other parts of the sandgrouse body in that they show the conventional structural array of barbs and barbules with their characteristic water-repelling properties. The structural parameter (r+d)jr for both male and female rates between 5-5 and 6-2 with slightly higher values within this range for the dorsal side. This result is in line with similar data on other terrestrial birds, indicating an effective water repellency without the necessity of preventing water penetration (Rijke, 1970). -

Sandgrouserefs Ver1.0.Pdf
Introduction I have endeavoured to keep typos, errors, omissions etc in this list to a minimum, however when you find more I would be grateful if you could mail the details during 2016 & 2017 to: [email protected]. Please note that this and other Reference Lists I have compiled are not exhaustive and are best employed in conjunction with other sources. Grateful thanks to Killian Mullarney for the cover images. All images © the photographer. Joe Hobbs Index The general order of species follows the International Ornithologists' Union World Bird List (Gill, F. & Donsker, D. (eds.) 2016. IOC World Bird List. Available from: http://www.worldbirdnames.org/ [version 6.1 accessed February 2016]). Version Version 1.0 (May 2016). Cover Main image: Chestnut-bellied and Spotted Sandgrouse. Near Thumrayt, Oman. 3rd November 2008. Picture by Killian Mullarney. Vignette: Spotted Sandgrouse. Near Thumrayt, Oman. 3rd November 2008. Picture by Killian Mullarney. Species Page No. Black-bellied Sandgrouse [Pterocles orientalis] 6 Black-faced Sandgrouse [Pterocles decoratus] 8 Burchell's Sandgrouse [Pterocles burchelli] 10 Chestnut-bellied Sandgrouse [Pterocles exustus] 5 Crowned Sandgrouse [Pterocles coronatus] 8 Double-banded Sandgrouse [Pterocles bicinctus] 9 Four-banded Sandgrouse [Pterocles quadricinctus] 9 Lichtenstein's Sandgrouse [Pterocles lichtensteinii] 8 Madagascar Sandgrouse [Pterocles personatus] 8 Namaqua Sandgrouse [Pterocles namaqua] 4 Painted Sandgrouse [Pterocles indicus] 9 Pallas's Sandgrouse [Syrrhaptes paradoxus] 3 Pin-tailed Sandgrouse [Pterocles alchata] 3 Spotted Sandgrouse [Pterocles senegallus] 6 Tibetan Sandgrouse [Syrrhaptes tibetanus] 2 Yellow-throated Sandgrouse [Pterocles gutturalis] 7 1 Relevant Publications Beaman, M. 1994. Palearctic birds: a checklist of the birds of Europe, North Africa and Asia north of the foothills of the Himalayas. -

A Study of the Ecology of the Namaqua Sandgrouse and Other Arid-Zone Birds
A STUDY OF THE ECOLOGY OF THE NAMAQUA SANDGROUSE AND OTHER ARID-ZONE BIRDS PENN LLOYD Thesis Presented for the Degree of DOCTOR OF PHILOSOPHY in the Percy Fitzpatrick I nstitute of African Ornithology UNIVERSITY OF CAPE TOWN February 1998' The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. "Part of facing up to the realities and complexity of nature is admilting that any approach we take will be incomplete, imperfect. provisional, experimental. The important thing is 10 try: Stephen Budiansky in Nature's Keepers And here I Iry I dedicate this thesis to my mother COLLEEN LLOYD for her many sacrifices to ensure my first-class education. and to DEKKER and SIKKIE STADLER whose hospitality and support kept me sane and made much of this study possible. I TABLE OF CONTENTS ABSTRACf ........................................................................................................................................................... ] ACKNOWLEDGEMENTS ................................................................................................................................. 0 CHAPTERl GENERAL INTRODUCfION ........................................................................................................................... -

Coos, Booms, and Hoots: the Evolution of Closed-Mouth Vocal Behavior in Birds
ORIGINAL ARTICLE doi:10.1111/evo.12988 Coos, booms, and hoots: The evolution of closed-mouth vocal behavior in birds Tobias Riede, 1,2 Chad M. Eliason, 3 Edward H. Miller, 4 Franz Goller, 5 and Julia A. Clarke 3 1Department of Physiology, Midwestern University, Glendale, Arizona 85308 2E-mail: [email protected] 3Department of Geological Sciences, The University of Texas at Austin, Texas 78712 4Department of Biology, Memorial University, St. John’s, Newfoundland and Labrador A1B 3X9, Canada 5Department of Biology, University of Utah, Salt Lake City 84112, Utah Received January 11, 2016 Accepted June 13, 2016 Most birds vocalize with an open beak, but vocalization with a closed beak into an inflating cavity occurs in territorial or courtship displays in disparate species throughout birds. Closed-mouth vocalizations generate resonance conditions that favor low-frequency sounds. By contrast, open-mouth vocalizations cover a wider frequency range. Here we describe closed-mouth vocalizations of birds from functional and morphological perspectives and assess the distribution of closed-mouth vocalizations in birds and related outgroups. Ancestral-state optimizations of body size and vocal behavior indicate that closed-mouth vocalizations are unlikely to be ancestral in birds and have evolved independently at least 16 times within Aves, predominantly in large-bodied lineages. Closed-mouth vocalizations are rare in the small-bodied passerines. In light of these results and body size trends in nonavian dinosaurs, we suggest that the capacity for closed-mouth vocalization was present in at least some extinct nonavian dinosaurs. As in birds, this behavior may have been limited to sexually selected vocal displays, and hence would have co-occurred with open-mouthed vocalizations. -

Common Birds of Namibia and Botswana 1 Josh Engel
Common Birds of Namibia and Botswana 1 Josh Engel Photos: Josh Engel, [[email protected]] Integrative Research Center, Field Museum of Natural History and Tropical Birding Tours [www.tropicalbirding.com] Produced by: Tyana Wachter, R. Foster and J. Philipp, with the support of Connie Keller and the Mellon Foundation. © Science and Education, The Field Museum, Chicago, IL 60605 USA. [[email protected]] [fieldguides.fieldmuseum.org/guides] Rapid Color Guide #584 version 1 01/2015 1 Struthio camelus 2 Pelecanus onocrotalus 3 Phalacocorax capensis 4 Microcarbo coronatus STRUTHIONIDAE PELECANIDAE PHALACROCORACIDAE PHALACROCORACIDAE Ostrich Great white pelican Cape cormorant Crowned cormorant 5 Anhinga rufa 6 Ardea cinerea 7 Ardea goliath 8 Ardea pupurea ANIHINGIDAE ARDEIDAE ARDEIDAE ARDEIDAE African darter Grey heron Goliath heron Purple heron 9 Butorides striata 10 Scopus umbretta 11 Mycteria ibis 12 Leptoptilos crumentiferus ARDEIDAE SCOPIDAE CICONIIDAE CICONIIDAE Striated heron Hamerkop (nest) Yellow-billed stork Marabou stork 13 Bostrychia hagedash 14 Phoenicopterus roseus & P. minor 15 Phoenicopterus minor 16 Aviceda cuculoides THRESKIORNITHIDAE PHOENICOPTERIDAE PHOENICOPTERIDAE ACCIPITRIDAE Hadada ibis Greater and Lesser Flamingos Lesser Flamingo African cuckoo hawk Common Birds of Namibia and Botswana 2 Josh Engel Photos: Josh Engel, [[email protected]] Integrative Research Center, Field Museum of Natural History and Tropical Birding Tours [www.tropicalbirding.com] Produced by: Tyana Wachter, R. Foster and J. Philipp, -

Columbiformes ~ Pterocliformes ~ Mesitornithiformes
Birds of the World part 2 Galloanseres, Neoaves: Columbea NEOGNATHAE (the rest of the birds!): Galloanseres • ORDER ANSERIFORMES – waterfowl • Family Anhimidae – screamers (3 species) • Family Anseranatidae – magpie goose (1 species) • Family Anatidae – ducks, geese, and swans (173 species) • ORDER GALLIFORMES – landfowl • Family Megapodiidae – megapodes (21 species) • Family Cracidae – chachalacas, curassows, and guans (55 species) • Family Numididae – guineafowl (6 species) • Family Odontophoridae – New World quail (34 species) • Family Phasianidae – pheasants and allies (183 species) NEOGNATHAE : Neoaves (the rest of the birds!): COLUMBEA • ORDER PODICIPEDIFORMES – Family Podicipedidae – grebes (23 species) • ORDER PHOENICOPTERIFORMES – Family Phoenicopteridae – flamingos (6 species) • ORDER COLUMBIFORMES – Family Columbidae – pigeons and doves (334 species) • ORDER PTEROCLIDIFORMES – Family Pteroclididae – sandgrouse (16 species) • ORDER MESITORNITHIFORMES – Family Mesitornithidae – mesites (3 species) NEOGNATHAE : Galloanseres • ORDER ANSERIFORMES – waterfowl • Family Anhimidae – screamers (3 species) • Family Anseranatidae – magpie goose (1 species) • Family Anatidae – ducks, geese, and swans (173 species) • ORDER GALLIFORMES – landfowl • Family Megapodiidae – megapodes (21 species) • Family Cracidae – chachalacas, curassows, and guans (55 species) • Family Numididae – guineafowl (6 species) • Family Odontophoridae – New World quail (34 species) • Family Phasianidae – pheasants and allies (183 species) southern or crested screamer -
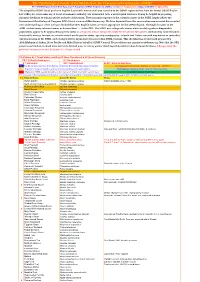
Simplified-ORL-2019-5.1-Final.Pdf
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa, Part F: Simplified OSME Region List (SORL) version 5.1 August 2019. (Aligns with ORL 5.1 July 2019) The simplified OSME list of preferred English & scientific names of all taxa recorded in the OSME region derives from the formal OSME Region List (ORL); see www.osme.org. It is not a taxonomic authority, but is intended to be a useful quick reference. It may be helpful in preparing informal checklists or writing articles on birds of the region. The taxonomic sequence & the scientific names in the SORL largely follow the International Ornithological Congress (IOC) List at www.worldbirdnames.org. We have departed from this source when new research has revealed new understanding or when we have decided that other English names are more appropriate for the OSME Region. The English names in the SORL include many informal names as denoted thus '…' in the ORL. The SORL uses subspecific names where useful; eg where diagnosable populations appear to be approaching species status or are species whose subspecies might be elevated to full species (indicated by round brackets in scientific names); for now, we remain neutral on the precise status - species or subspecies - of such taxa. Future research may amend or contradict our presentation of the SORL; such changes will be incorporated in succeeding SORL versions. This checklist was devised and prepared by AbdulRahman al Sirhan, Steve Preddy and Mike Blair on behalf of OSME Council. Please address any queries to [email protected]. -

The Origin and Diversification of Birds
Current Biology Review The Origin and Diversification of Birds Stephen L. Brusatte1,*, Jingmai K. O’Connor2,*, and Erich D. Jarvis3,4,* 1School of GeoSciences, University of Edinburgh, Grant Institute, King’s Buildings, James Hutton Road, Edinburgh EH9 3FE, UK 2Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China 3Department of Neurobiology, Duke University Medical Center, Durham, NC 27710, USA 4Howard Hughes Medical Institute, Chevy Chase, MD 20815, USA *Correspondence: [email protected] (S.L.B.), [email protected] (J.K.O.), [email protected] (E.D.J.) http://dx.doi.org/10.1016/j.cub.2015.08.003 Birds are one of the most recognizable and diverse groups of modern vertebrates. Over the past two de- cades, a wealth of new fossil discoveries and phylogenetic and macroevolutionary studies has transformed our understanding of how birds originated and became so successful. Birds evolved from theropod dino- saurs during the Jurassic (around 165–150 million years ago) and their classic small, lightweight, feathered, and winged body plan was pieced together gradually over tens of millions of years of evolution rather than in one burst of innovation. Early birds diversified throughout the Jurassic and Cretaceous, becoming capable fliers with supercharged growth rates, but were decimated at the end-Cretaceous extinction alongside their close dinosaurian relatives. After the mass extinction, modern birds (members of the avian crown group) explosively diversified, culminating in more than 10,000 species distributed worldwide today. Introduction dinosaurs Dromaeosaurus albertensis or Troodon formosus.This Birds are one of the most conspicuous groups of animals in the clade includes all living birds and extinct taxa, such as Archaeop- modern world.