Seed Dispersal by a Generalist Duck
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Understanding Mikvah
Understanding Mikvah An overview of Mikvah construction Copyright © 2001 by Rabbi S. Z. Lesches permission & comments: (514) 737-6076 4661 Van Horne, Suite 12 Montreal P.Q. H3W 1H8 Canada National Library of Canada Cataloguing in Publication Data Lesches, Schneur Zalman Understanding mikvah : an overview of mikvah construction ISBN 0-9689146-0-8 1. Mikveh--Design and construction. 2. Mikveh--History. 3. Purity, Ritual--Judaism. 4. Jewish law. I. Title. BM703.L37 2001 296.7'5 C2001-901500-3 v"c CONTENTS∗ FOREWORD .................................................................... xi Excerpts from the Rebbe’s Letters Regarding Mikvah....13 Preface...............................................................................20 The History of Mikvaos ....................................................25 A New Design.............................................................27 Importance of a Mikvah....................................................30 Building and Planning ......................................................33 Maximizing Comfort..................................................34 Eliminating Worry ......................................................35 Kosher Waters ...................................................................37 Immersing in a Spring................................................37 Oceans..........................................................................38 Rivers and Lakes .........................................................38 Swimming Pools .........................................................39 -

Lulav-And-Etrog-Instructions.Pdf
אֶּתְ רֹוג לּולָב LULAV AND ETROG: THE FOUR SPECIES What they are and what to do with them INTRODUCTION The commandment regarding the four species (of the lulav and etrog) is found in the Torah. After discussing the week-long Sukkot festival, specific instructions for how to celebrate the holiday are given. Leviticus 23:40 instructs: םּולְקַחְתֶּ לָכֶּם בַּיֹוםהָרִ אׁשֹון פְרִ י עֵץ הָדָרכַפֹּת תְ מָרִ ים וַעֲנַף עֵץ־עָבֹּת וְעַרְ בֵי־נָחַל ּושְ מַחְתֶּ ם לִפְ נֵי ה' אֱֹלהֵיכֶּם ׁשִבְ עַת יָמִ ים “On the first day you shall take the product of hadar trees, branches of palm trees, boughs of leafy trees, and willows of the brook, and you shall rejoice before Adonai your God seven days." These are the four species that form the lulav and etrog. The four species are waved in the synagogue as part of the service during the holiday of Sukkot. Traditionally, they are not waved on Shabbat because bringing these items to the synagogue would violate the prohibition against carrying. Some liberal synagogues do wave the lulav and etrog on Shabbat. While it is customary for each individual to have a lulav and etrog, many synagogues leave some sets in the synagogue sukkah for the use of their members. The lulav and etrog may also be waved at home. Below you will find some basic information about the lulav and etrog, reprinted with permission from The Jewish Catalogue: A Do-It-Yourself Kit, edited by Richard Siegel, Michael Strassfeld and Sharon Strassfeld, published by the Jewish Publication Society. HOW THE FOUR PARTS FIT TOGETHER The lulav is a single palm branch and occupies the central position in the grouping. -

Kol Hamishtakker
Kol Hamishtakker Ingredients Kol Hamishtakker Volume III, Issue 5 February 27, 2010 The Student Thought Magazine of the Yeshiva 14 Adar 5770 University Student body Paul the Apostle 3 Qrum Hamevaser: The Jewish Thought Magazine of the Qrum, by the Qrum, and for the Qrum Staph Dover Emes 4 Reexamining the Halakhot of Maharat-hood Editors-in-Chief The Vatikin (in Italy) 4 The End of an Era Sarit “Mashiah” Bendavid Shaul “The Enforcer” Seidler-Feller Ilana Basya “Tree Pile” 5 Cherem Against G-Chat Weitzentraegger Gadish Associate Editors Ilana “Good Old Gad” Gadish Some Irresponsible Feminist 7 A Short Proposal for Female Rabbis Shlomo “Yam shel Edmond” Zuckier (Pseudonym: Stephanie Greenberg) Censorship Committee Jaded Narrative 7 How to Solve the Problem of Shomer R’ M. Joel Negi’ah and Enjoy Life Better R’ Eli Baruch Shulman R’ Mayer Twersky Nathaniel Jaret 8 The Shiddukh Crisis Reconsidered: A ‘Plu- ral’istic Approach Layout Editor Menachem “Still Here” Spira Alex Luxenberg 9 Anu Ratzim, ve-Hem Shkotzim: Keeping with Menachem Butler Copy Editor Benjamin “Editor, I Barely Even Know Her!” Abramowitz Sheketah Akh Katlanit 11 New Dead Sea Sect Found Editors Emeritus [Denied Tenure (Due to Madoff)] Alex Luxenberg 13 OH MY G-DISH!: An Interview with Kol R’ Yona Reiss Hamevaser Associate Editor Ilana Gadish Alex Sonnenwirth-Ozar Friedrich Wilhelm Benjamin 13 Critical Studies: The Authorship of the Staph Writers von Rosenzweig “Documentary Hypothesis” Wikipedia Arti- A, J, P, E, D, and R Berkovitz cle Chaya “Peri Ets Hadar” Citrin Rabbi Shalom Carmy 14 Torah u-Media: A Survey of Stories True, Jake “Gush Guy” Friedman Historical, and Carmesian Nicole “Home of the Olympics” Grubner Nate “The Negi’ah Guy” Jaret Chaya Citrin 15 Kol Hamevater: A New Jewish Thought Ori “O.K.” Kanefsky Magazine of the Yeshiva University Student Alex “Grand Duchy of” Luxenberg Body Emmanuel “Flanders” Sanders Yossi “Chuent” Steinberger Noam Friedman 15 CJF Winter Missions Focus On Repairing Jonathan “’Lil ‘Ling” Zirling the World Disgraced Former Staph Writers Dr. -

CONGREGATION BETH YESHURUN INVITATION to JUDAISM COURSE CURRICULUM – 5781 (2020 – 2021) (As of 08-17-20)
CONGREGATION BETH YESHURUN INVITATION TO JUDAISM COURSE CURRICULUM – 5781 (2020 – 2021) (As of 08-17-20) # and Date TOPIC for 1st Hr. (9:00-10:00) [2nd Hr. (10:00-11:00) is Hebrew class] 1 Sept. 6 Conversion to Judaism - Overview [No Hebrew class] 2 Sept 13 High Holy Days and Sukkot [No Hebrew class] ⁂ Sept. 19-20 Rosh Hashanah begins Friday night Sept. 18 – Sunday night Sept. 20 ⁂ Sept. 28 Yom Kippur starts Sunday night Sept. 27 - Monday night Sept. 28 3 Sept. 29 Sukkot and the Jewish Calendar (Tuesday evening at 7:00) ⁂ Oct. 3 Sukkot begins Friday night Oct. 2 through Friday Oct. 9. Then Shemini Atzeret and Simhat Torah Friday night Oct. 9 – Sunday night Oct. 11 4 Oct. 18 Introduction to Prayers – Structure of Siddur, overview of services [Hebrew class starts this week at 10:00-11:00] 5 Oct. 25 Shabbat 6 Nov. 1 Overview of J. History, Classic J. Texts, J. Book List [visit ERJCC website] ⁂ Nov. 1 - Nov. 19 Virtual Book and Arts Festival at JCC 7 Nov. 8 Beliefs: God, Revelation, Torah, Mitzvot (cf Christianity) 8 Nov. 15 Beliefs: Life After Death/Messiah/Resurrection (cf Christianity) 9 Nov. 22 Beliefs: The Problem of Evil & Reward and Punishment (cf Christianty) 10 Dec. 6 Hanukkah (cf Christmas) ⁂ Dec. 10 - Dec. 18 Hanukkah (1st candle Dec. 10, 8th candle Dec. 17) 11 Dec. 13 Prayers: Shema & its Blessings (incl. Mezuzah/tzitzit/tefillin) 12 Dec. 20 Prayers – Amidah 13 Jan. 10 Kashrut 14 Jan. 17 Ethics – Tzedakah/Gemilut Hasadim 15 Jan. 24 Ethics – Honoring Parents/Aged, Bikur Holim 16 Jan. -
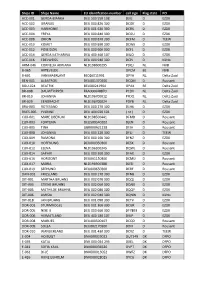
Ships ID Ships Name EU Identifcation Number Call Sign Flag State PO ACC
Ships ID Ships Name EU identifcation number call sign Flag state PO ACC-001 GERDA-BIANKA DEU 500 550 108 DJIG D EZDK ACC-002 URANUS DEU 000 820 300 DCGK D EZDK ACC-003 HARMONIE DEU 001 630 300 DCRK D EZDK ACC-004 FREYA DEU 000 840 300 DCGU D EZDK ACC-008 ORION DEU 000 870 300 DCFM D TEEW ACC-010 KOMET DEU 000 890 300 DCWK D EZDK ACC-012 POSEIDON DEU 000 900 300 DCFL D EZDK ACC-014 GERDA KATHARINA DEU 400 460 107 DIUO D EZDK ACC-016 EDELWEISS DEU 000 940 300 DCPJ D KüNo ARM-046 CORNELIA ADRIANA NLD198600295 PDQJ NL NVB B-065 ARTEVELDE OPCM BE NVB B-601 VAN MAERLANT BE026011991 OPYA NL Delta Zuid BEN-001 ALBATROS DEU001370300 DCQM D Rousant BOU-024 BEATRIX BEL000241964 OPAX BE Delta Zuid BR-008 DAUWTRIPPER FRA000648870 PCDV NL Delta Zuid BR-010 JOHANNA NLD196700012 PFDQ NL Delta Zuid BR-029 EENDRACHT NLD196700024 PDYB NL Delta Zuid BRA-003 ROTESAND DEU 000 270 300 DLHX D EZDK BUES-005 YVONNE DEU 404 020 101 ( nc ) D EZDK CUX-001 MARE LIBERUM NLD198500441 DFMD D Rousant CUX-003 FORTUNA DEU500540102 DJEN D Rousant CUX-005 TINA GBR000A21228 DFJH D Rousant CUX-008 JOHANNA DEU 000 220 300 DFLJ D TEEW CUX-009 RAMONA DEU 000 160 300 DFNZ D EZDK CUX-010 HOFFNUNG DEU000350300 DESX D Rousant CUX-012 ELENA NLD196300345 DQWL D Rousant CUX-014 SAPHIR DEU 000 360 300 DFAX D EZDK CUX-016 HORIZONT DEU001150300 DCMU D Rousant CUX-017 MARIA NLD196900388 DJED D Rousant CUX-019 SEEHUND DEU000650300 DERF D Rousant DAN-001 FRIESLAND DEU 000 170 300 DFNB D EZDK DIT-001 MARTHA BRUHNS DEU 002 070 300 DQQJ D EZDK DIT-003 STIENE BRUHNS DEU 002 060 300 DQNX D EZDK -

Building Our Spiritual Community
UPCOMING EVENTS, ACTIVITIES AND SPECIAL ANNOUNCEMENTS Building Our Spiritual Community FRIDAY, OCTOBER 6 – 17 TISHRI 6 PM - Minha/Kabbalat Shabbat/Ma’ariv 6:16 PM - Candle Lighting SATURDAY, OCTOBER 7 – 17 TISHRI 9 AM - Kol Tefilah – Morning Service Bar Mitzvah: JORDAN PEARL, son of Melissa & David Pearl 9:45 AM - Parasha and Pop’ems – Grades 3-7 10:45 AM - Parashat Hashavua – Adult Torah Study 10:45 AM - Teens and Torah 6:15 PM - Minha/Seudah Shlishit/Ma’ariv/Havdalah 7:12 PM - Shabbat ends New Procedures for the Mi Shebeirach List In an effort to better serve the needs of our congregation, we are changing the way we maintain and update our Mi Shebeirach list (the list of people who have requested prayers for healing). To make sure our list is as current as possible, we are building a new list from scratch. So even if you have previously asked that a name be placed on this list, we ask that you call or email and request that person’s name be included on the list. Moving forward, names will be kept on the list for four weeks and then removed unless you, or a member of our Clergy team specifically request that they remain longer. To have a name added to the list or to update the status of the person named, please call or email Laurie Albert in the Clergy office at 610-667-5000 x111 or [email protected]. SUKKOT/SIMHAT TORAH AT HAR ZION Following services on the evenings of October 4, 5 and 6, Kiddush will be in the Maurice A. -

CCAR Journal the Reform Jewish Quarterly
CCAR Journal The Reform Jewish Quarterly Halachah and Reform Judaism Contents FROM THE EDITOR At the Gates — ohrgJc: The Redemption of Halachah . 1 A. Brian Stoller, Guest Editor ARTICLES HALACHIC THEORY What Do We Mean When We Say, “We Are Not Halachic”? . 9 Leon A. Morris Halachah in Reform Theology from Leo Baeck to Eugene B . Borowitz: Authority, Autonomy, and Covenantal Commandments . 17 Rachel Sabath Beit-Halachmi The CCAR Responsa Committee: A History . 40 Joan S. Friedman Reform Halachah and the Claim of Authority: From Theory to Practice and Back Again . 54 Mark Washofsky Is a Reform Shulchan Aruch Possible? . 74 Alona Lisitsa An Evolving Israeli Reform Judaism: The Roles of Halachah and Civil Religion as Seen in the Writings of the Israel Movement for Progressive Judaism . 92 David Ellenson and Michael Rosen Aggadic Judaism . 113 Edwin Goldberg Spring 2020 i CONTENTS Talmudic Aggadah: Illustrations, Warnings, and Counterarguments to Halachah . 120 Amy Scheinerman Halachah for Hedgehogs: Legal Interpretivism and Reform Philosophy of Halachah . 140 Benjamin C. M. Gurin The Halachic Canon as Literature: Reading for Jewish Ideas and Values . 155 Alyssa M. Gray APPLIED HALACHAH Communal Halachic Decision-Making . 174 Erica Asch Growing More Than Vegetables: A Case Study in the Use of CCAR Responsa in Planting the Tri-Faith Community Garden . 186 Deana Sussman Berezin Yoga as a Jewish Worship Practice: Chukat Hagoyim or Spiritual Innovation? . 200 Liz P. G. Hirsch and Yael Rapport Nursing in Shul: A Halachically Informed Perspective . 208 Michal Loving Can We Say Mourner’s Kaddish in Cases of Miscarriage, Stillbirth, and Nefel? . 215 Jeremy R. -

Instructions for the Use of the Lulav and Esrog 1. in Your Right Hand
Instructions for the Use of the Lulav and Esrog 1. In your right hand, take the Lulav (palm branch) – together with the hadassim (3 myrtle branches) on it’s right and the Aravos (willows – 2 branches) on it’s left, with the spine of the Lulav (darker green column) facing you. In your left hand, take the Esrog (citron fruit), with the stem (wider part) facing upwards. (A left handed person should take the Lulav in his left hand the the Esrog in his right) 2. While standing and facing east say the bracha (blessing) “Al Netilas Lulav” (Artscroll page 630). On the first day of Sukkos say the bracha (blessing) “Shehecheyanu.” After the bracha(s) turn the Esrog over (so that the stem faces downward) and hold it close to the Lulav to that there is no separate between them Wave the species towards the six point of the earth, in the following sequence: Straight ahead To the right Behind you To the left Upwards Downwards Note: You do not have to wave the Lulav forcefully – shaking it slightly to rustle the leaves is sufficient. It should be shaken three times in each direction before moving on to the next direction. When waving downward you should lower only your hands while the Lulav and other species remain in an upright position. If by mistake you took the Esrog with the right hand and the Lulav with the left hand you should shake them again without saying the bracha(s). 3. The four species are also waved during the recital of Hallel (Artscroll p. -

Sukkot in the Torah דַּבֵּ ר אֶל בְּנֵי יִשְׂרָאֵל
BY RABBI ELAZAR MEISELS “For seven days you shall dwell in Sukkot… This is so that future generations will know Sukkot in the Torah that I had the Israelites live in booths when I brought them out of Egypt.” ּדַ ּבֵר ֶאל ּבְנֵי יִ ְש ׂרָ ֵאל לֵ ֹאמר ּ ַב ֲח ִמ ּׁ ָשה עָ ָש ׂר יוֹם לַ ֹחדֶשׁ ַה ּׁ ְש ִב ִיעי ַה ּזֶה ַחג ַה ּסֻכּוֹת Rabbi Eliezer holds that these booths were the Clouds of Glory which encircled ׁ ִשבְעַת יָ ִמים לַ ֹידוָד: ּ ַביּוֹם ָהרִאשׁוֹן ִמ ְקרָא ֹקדֶשׁ ּכָל ְמלֶאכֶת עֲ ֹבדָה ֹלא ַתעֲשׂוּ: and protected us throughout our stay in ׁ ִשבְעַת יָ ִמים ּ ַת ְקרִיבוּ ִא ּׁ ֶשה לַ ֹידוָד ּ ַביּוֹם ַה ּׁ ְש ִמינִי ִמ ְקרָא ֹקדֶשׁ יִ ְהיֶה לָכֶם the desert. Rabbi Akiva explains that the verse refers to the actual tents in which וְ ִה ְקרַבְ ּ ֶתם ִא ּׁ ֶשה לַ ֹידוָד עֲצֶרֶת ִהוא ּכָל ְמלֶאכֶת עֲ ֹבדָה ֹלא ַתעֲשׂוּ: ֵא ּלֶה מוֹעֲדֵי .we lived while sojourning the desert יְ ֹדוָד ֲא ׁ ֶשר ּ ִת ְקרְאוּ ֹא ָתם ִמ ְקרָ ֵאי ֹקדֶשׁ לְ ַה ְקרִיב ִא ׁ ֶשּה לַ ֹידוָד ֹעלָה ִוּמנְ ָחה זֶ ַבח Talmud, Tractate Sukkah 11b וּנְ ָס ִכים ּדְ ַבר יוֹם ּבְיוֹמוֹ: ִמ ּלְ ַבד ׁ ַש ּבְ ֹתת יְ ֹדוָד ִוּמ ּלְ ַבד ַמ ּ ְת ֵנוֹתיכֶם ִוּמ ּלְ ַבד ּכָל What is the significance of the tents נִדְרֵיכֶם ִוּמ ּלְ ַבד ּכָל נִדְ ֹב ֵתיכֶם ֲא ׁ ֶשר ּ ִת ּ ְתנוּ לַ ֹידוָד: ַאךְ ּ ַב ֲח ִמ ּׁ ָשה עָ ָש ׂר יוֹם לַ ֹחדֶשׁ in the desert that they deserve such a serious commemoration? The Sukkah ַה ּׁ ְש ִב ִיעי ּבְ ָא ְס ּפְכֶם ֶאת ּ ְת ַבוּאת ָה ָארֶץ ּ ָת ֹחגּוּ ֶאת ַחג יְ ֹדוָד ׁ ִשבְעַת יָ ִמים ּ ַביּוֹם reminds us of the great faith of the Jewish ָהרִאשׁוֹן ׁ -

Pardes Companion
The Sukkot Companion is dedicated in honor of Sherwin Pomerantz Chairperson, Pardes Israel Board of Directors, as we celebrate his 80th birthday Pardes Sukkot Companion The Shadow of Faith Mike Feuer Rabbi Mike Feuer teaches Striving for the Divine, Rav Kook, and Jewish History at Pardes. There are two critical elements that define the roof We find the first mention of the word emunah in of our sukkah: It must provide more shade than light, reference to Moshe’s hands as he holds them up in and we must see the stars through its branches. battle against Amalek, the biblical embodiment of doubt: “Whenever Moshe held up his hand, Israel This interplay between light and darkness, and prevailed; but whenever he let down his hand, the lesson that it offers, is why the Zohar calls the Amalek prevailed.” Despite Moshe’s weariness, sukkah, tzilah d’mheimnuta, “the shade of emunah.” the Torah tells us that “his hands were emunah Emunah is a difficult word to translate, and its until the sun set” (Exodus 17:12). The Hizkuni meaning is an essential message of the holiday. explains that emunah here refers to that which The origin of the term is found with Avraham when stands fast and does not weaken, and translates he looks up at the heavens and sees a barren the verse as “Moshe’s hands remained steadfast.” future. In his pain he cries out to God, who replies, Only days after the exodus, the people had brought “Look toward heaven and count the stars…so shall the punishment of war with Amalek by asking, “Is your offspring be.” It is an impossible promise God among us or not?” (Exodus 17:7). -

Developmental Therapeutics Immunotherapy
DEVELOPMENTAL THERAPEUTICS—IMMUNOTHERAPY 2500 Oral Abstract Session Clinical activity of systemic VSV-IFNb-NIS oncolytic virotherapy in patients with relapsed refractory T-cell lymphoma. Joselle Cook, Kah Whye Peng, Susan Michelle Geyer, Brenda F. Ginos, Amylou C. Dueck, Nandakumar Packiriswamy, Lianwen Zhang, Beth Brunton, Baskar Balakrishnan, Thomas E. Witzig, Stephen M Broski, Mrinal Patnaik, Francis Buadi, Angela Dispenzieri, Morie A. Gertz, Leif P. Bergsagel, S. Vincent Rajkumar, Shaji Kumar, Stephen J. Russell, Martha Lacy; Mayo Clinic, Rochester, MN; The Ohio State University, Columbus, OH; Mayo Clinic, Scottsdale, AZ; Division of Hematology, Mayo Clinic, Roches- ter, MN; Vyriad and Mayo Clinic, Rochester, MN Background: Oncolytic virotherapy is a novel immunomodulatory therapeutic approach for relapsed re- fractory hematologic malignancies. The Indiana strain of Vesicular Stomatitis Virus was engineered to encode interferon beta (IFNb) and sodium iodine symporter (NIS) to produce VSV-IFNb-NIS. Virally en- coded IFNb serves as an index of viral proliferation and enhances host anti-tumor immunity. NIS was in- serted to noninvasively assess viral biodistribution using SPECT/PET imaging. We present the results of the phase 1 clinical trial NCT03017820 of systemic administration of VSV-IFNb-NIS among patients (pts) with relapsed refractory Multiple Myeloma (MM), T cell Lymphoma (TCL) and Acute myeloid Leu- 9 kemia (AML). Methods: VSV-IFNb-NIS was administered at 5x10 TCID50 (50% tissue culture infec- 11 tious dose) dose level 1 to dose level 4, 1.7x10 TCID50. The primary objective was to determine the maximum tolerated dose of VSV-IFNb-NIS as a single agent. Secondary objectives were determination of safety profile and preliminary efficacy of VSV-IFNb-NIS. -

Programme Book
10th European Public Health Conference OVERVIEW PROGRAMME Sustaining resilient WEDNESDAY 1 NOVEMBER THURSDAY 2 NOVEMBER FRIDAY 3 NOVEMBER SATURDAY 4 NOVEMBER 09:00 – 12:30 09:00 – 10:30 08:30 - 09:30 08:30 - 10:00 Pre-conferences Parallel sessions 1 Parallel sessions 4 Parallel session 8 09:40 - 10:40 10:10 - 11:10 and healthy communities Plenary 3 Parallel session 9 10:30 – 11:00 10:30 – 11:00 10:40 – 11:10 11:10 – 11:40 Coffee/tea Coffee/tea Coffee/tea Coffee/tea 11th European Public Health Conference 2018 12th European Public Health Conference 2019 11:00 – 12:00 11:10 - 12:10 11:40 - 13:10 Ljubljana, Slovenia Marseille, France PROGRAMME Plenary 1 Parallel sessions 5 Parallel session 10 12:30 – 13:30 12:00 – 13:15 12:10 – 13:40 13:10 – 14:10 Lunch for pre-conference Lunch – Lunch symposiums and Lunch – Lunch symposiums Lunch – Join the Networks delegates only Join the Networks and Join the Networks 13:30 – 17:00 13:15 - 14:15 13:40 - 14:40 14:10 - 15:10 Pre-conferences Plenary 2 Plenary 4 Plenary 5 14:25 – 15:25 14:50-15:50 15:10 – 16:00 Parallel sessions 2 Parallel sessions 6 Closing Ceremony Winds of Change: towards new ways Building bridges for solidarity 15:00 – 15:30 15:25 – 15:55 15:50 – 16:20 of improving public health in Europe and public health Coffee/tea Coffee/tea Coffee/tea 17:00 16:05 – 17:05 16:20 - 17:50 28 November - 1 December 2018 20 - 23 November 2019 End of Programme Parallel sessions 3 Parallel sessions 7 Cankarjev Dom, Ljubljana Marseille Chanot, Marseille 17:15 - 18:00 Opening Ceremony @EPHConference #EPH2018