Artificial Hybridization Between U.S. Native Ruellia Caroliniensis And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Paynes Creek Historic State Park
Paynes Creek Historic State Park Approved Plan Unit Management Plan STATE OF FLORIDA DEPARTMENT OF ENVIRONMENTAL PROTECTION Division of Recreation and Parks December 16, 2016 TABLE OF CONTENTS INTRODUCTION ...................................................................................1 PURPOSE AND SIGNIFICANCE OF THE PARK ....................................... 1 Park Significance ................................................................................1 PURPOSE AND SCOPE OF THE PLAN..................................................... 2 MANAGEMENT PROGRAM OVERVIEW ................................................... 8 Management Authority and Responsibility .............................................. 8 Park Management Goals ...................................................................... 8 Management Coordination ................................................................... 9 Public Participation ..............................................................................9 Other Designations .............................................................................9 RESOURCE MANAGEMENT COMPONENT INTRODUCTION ................................................................................. 11 RESOURCE DESCRIPTION AND ASSESSMENT..................................... 12 Natural Resources ............................................................................. 12 Topography .................................................................................. 12 Geology ...................................................................................... -

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Natural Communities of Michigan: Classification and Description
Natural Communities of Michigan: Classification and Description Prepared by: Michael A. Kost, Dennis A. Albert, Joshua G. Cohen, Bradford S. Slaughter, Rebecca K. Schillo, Christopher R. Weber, and Kim A. Chapman Michigan Natural Features Inventory P.O. Box 13036 Lansing, MI 48901-3036 For: Michigan Department of Natural Resources Wildlife Division and Forest, Mineral and Fire Management Division September 30, 2007 Report Number 2007-21 Version 1.2 Last Updated: July 9, 2010 Suggested Citation: Kost, M.A., D.A. Albert, J.G. Cohen, B.S. Slaughter, R.K. Schillo, C.R. Weber, and K.A. Chapman. 2007. Natural Communities of Michigan: Classification and Description. Michigan Natural Features Inventory, Report Number 2007-21, Lansing, MI. 314 pp. Copyright 2007 Michigan State University Board of Trustees. Michigan State University Extension programs and materials are open to all without regard to race, color, national origin, gender, religion, age, disability, political beliefs, sexual orientation, marital status or family status. Cover photos: Top left, Dry Sand Prairie at Indian Lake, Newaygo County (M. Kost); top right, Limestone Bedrock Lakeshore, Summer Island, Delta County (J. Cohen); lower left, Muskeg, Luce County (J. Cohen); and lower right, Mesic Northern Forest as a matrix natural community, Porcupine Mountains Wilderness State Park, Ontonagon County (M. Kost). Acknowledgements We thank the Michigan Department of Natural Resources Wildlife Division and Forest, Mineral, and Fire Management Division for funding this effort to classify and describe the natural communities of Michigan. This work relied heavily on data collected by many present and former Michigan Natural Features Inventory (MNFI) field scientists and collaborators, including members of the Michigan Natural Areas Council. -

10 Easy Wildflowers for Butterflies and Bees Tips and Terms
10 Easy Wildflowers for Butterflies and Bees Tips and Terms Selection Glossary of helpful terms It may take a while to understand your landscape’s soil and drainage conditions. If Anther: pollen-bearing part of the stamen your wildflowers don’t succeed, try again, maybe with different species. Remember, Axil: upper angle between the stem and success depends on using the right plant in the right place. leaf or other plant part Water Basal: forming or attached at the base Water plants thoroughly when planting, then water as needed until they are established Bract: modified leaf at the base of a flower and putting out new foliage. Once plants are established, irrigation should be needed Calyx: collective term for the sepals of a only during extended dry periods. Learn to recognize when plants look wilted and flower; typically a whorl that encloses water them then. Over-irrigation can cause fungus and rot, which can kill your the petals and protects the flower bud wildflowers. It can also cause them to grow too quickly, becoming more susceptible to pests and diseases, or too tall, requiring staking. Corolla: collective term for the petals of a flower Fertilizer Corona: petal-like structures arising from Native wildflowers should not need fertilizer. Applying fertilizer can produce plants that the corolla of some flowers to form a grow too quickly, which can lead them to become pest and disease prone, and too tall, crownlike ring requiring staking. Fertilizing also encourages weeds, which can easily out-compete Cultivar: horticultural variety of a wildflowers. naturally occurring species produced in cultivation by selective breeding Sustaining wildflowers If you want wildflowers to persist on their own in your landscape, you’ll need to allow for Deciduous: seasonal shedding of leaves; self-seeding, especially for annual or short-lived species. -

Cocoa Beach Maritime Hammock Preserve Management Plan
MANAGEMENT PLAN Cocoa Beach’s Maritime Hammock Preserve City of Cocoa Beach, Florida Florida Communities Trust Project No. 03 – 035 –FF3 Adopted March 18, 2004 TABLE OF CONTENTS SECTION PAGE I. Introduction ……………………………………………………………. 1 II. Purpose …………………………………………………………….……. 2 a. Future Uses ………….………………………………….…….…… 2 b. Management Objectives ………………………………………….... 2 c. Major Comprehensive Plan Directives ………………………..….... 2 III. Site Development and Improvement ………………………………… 3 a. Existing Physical Improvements ……….…………………………. 3 b. Proposed Physical Improvements…………………………………… 3 c. Wetland Buffer ………...………….………………………………… 4 d. Acknowledgment Sign …………………………………..………… 4 e. Parking ………………………….………………………………… 5 f. Stormwater Facilities …………….………………………………… 5 g. Hazard Mitigation ………………………………………………… 5 h. Permits ………………………….………………………………… 5 i. Easements, Concessions, and Leases …………………………..… 5 IV. Natural Resources ……………………………………………..……… 6 a. Natural Communities ………………………..……………………. 6 b. Listed Animal Species ………………………….…………….……. 7 c. Listed Plant Species …………………………..…………………... 8 d. Inventory of the Natural Communities ………………..………….... 10 e. Water Quality …………..………………………….…..…………... 10 f. Unique Geological Features ………………………………………. 10 g. Trail Network ………………………………….…..………..……... 10 h. Greenways ………………………………….…..……………..……. 11 i Adopted March 18, 2004 V. Resources Enhancement …………………………..…………………… 11 a. Upland Restoration ………………………..………………………. 11 b. Wetland Restoration ………………………….…………….………. 13 c. Invasive Exotic Plants …………………………..…………………... 13 d. Feral -
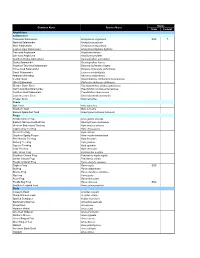
St. Joseph Bay Native Species List
Status Common Name Species Name State Federal Amphibians Salamanders Flatwoods Salamander Ambystoma cingulatum SSC T Marbled Salamander Ambystoma opacum Mole Salamander Ambystoma talpoideum Eastern Tiger Salamander Ambystoma tigrinum tigrinum Two-toed Amphiuma Amphiuma means One-toed Amphiuma Amphiuma pholeter Southern Dusky Salamander Desmognathus auriculatus Dusky Salamander Desmognathus fuscus Southern Two-lined Salamander Eurycea bislineata cirrigera Three-lined Salamander Eurycea longicauda guttolineata Dwarf Salamander Eurycea quadridigitata Alabama Waterdog Necturus alabamensis Central Newt Notophthalmus viridescens louisianensis Slimy Salamander Plethodon glutinosus glutinosus Slender Dwarf Siren Pseudobranchus striatus spheniscus Gulf Coast Mud Salamander Pseudotriton montanus flavissimus Southern Red Salamander Pseudotriton ruber vioscai Eastern Lesser Siren Siren intermedia intermedia Greater Siren Siren lacertina Toads Oak Toad Bufo quercicus Southern Toad Bufo terrestris Eastern Spadefoot Toad Scaphiopus holbrooki holbrooki Frogs Florida Cricket Frog Acris gryllus dorsalis Eastern Narrow-mouthed Frog Gastrophryne carolinensis Western Bird-voiced Treefrog Hyla avivoca avivoca Cope's Gray Treefrog Hyla chrysoscelis Green Treefrog Hyla cinerea Southern Spring Peeper Hyla crucifer bartramiana Pine Woods Treefrog Hyla femoralis Barking Treefrog Hyla gratiosa Squirrel Treefrog Hyla squirella Gray Treefrog Hyla versicolor Little Grass Frog Limnaoedus ocularis Southern Chorus Frog Pseudacris nigrita nigrita Ornate Chorus Frog Pseudacris -

Hydrologic Restoration Plan for the Tate's Hell State Forest
Tate’s Hell State Forest Hydrologic Restoration Plan Volume I Northwest Florida Water Management District 81 Water Management Drive, Havana, Florida 32333-4712 Florida Division of Forestry 290 Airport Road, Carrabelle, FL 32322 i GOVERNING BOARD George Roberts, Chair Panama City Philip McMillan, Vice Chair Blountstown Steve Ghazvini, Secretary/Treasurer Tallahassee Stephanie Bloyd Joyce Estes Jerry Pate Panama City Beach Eastpoint Pensacola Peter Antonacci Ralph Rish Tim Norris Tallahassee Port St. Joe Santa Rosa Beach Douglas E. Barr Executive Director For additional information, write or call: Northwest Florida Water Management District 81 Water Management Drive Havana, Florida 32333-4712 (850) 539-5999 DISTRICT OFFICES Headquarters 81 Water Management Drive Havana, FL 32333-4712 Telephone (850) 539-5999 Fax (850) 539-2777 Crestview 800 Hospital Drive Crestview, Florida 32539-7385 Telephone (850) 683-5044 Fax (850) 683-5050 Tallahassee The Delaney Center Building, Suite 2-D 2252 Killearn Center Boulevard Tallahassee, FL 32309-3573 Telephone (850) 921-2986 Fax (850) 921-3083 Pensacola (Field Office) 2261 West Nine Mile Road Pensacola, FL 32534-9416 Telephone (850) 484-5125 Fax (850) 484-5133 Marianna 4765 Pelt Street Marianna, FL 32446-6846 Telephone (850) 482-9522 Fax (850) 482-1376 Econfina (Field Office) 6418 E. Highway 20 Youngstown, FL 32466-3808 Telephone (850) 722-9919 Fax (850) 722-8982 Acknowledgements: This Hydrologic Restoration Plan is the result of the work and contributions of many individuals. At the Northwest Florida Water Management District, Ron Bartel, Director of the Resource Management Division, supervised this effort and provided oversight during the plan development process. We gratefully acknowledge Linda Chaisson, who participated in many of the field reviews along with Nick Wooten, Duncan Cairns, David Clayton, and John Crowe, who provided valuable guidance and suggestions regarding the plan‟s content. -

Mississippi Natural Heritage Program Special Plants - Tracking List -2018
MISSISSIPPI NATURAL HERITAGE PROGRAM SPECIAL PLANTS - TRACKING LIST -2018- Approximately 3300 species of vascular plants (fern, gymnosperms, and angiosperms), and numerous non-vascular plants may be found in Mississippi. Many of these are quite common. Some, however, are known or suspected to occur in low numbers; these are designated as species of special concern, and are listed below. There are 495 special concern plants, which include 4 non- vascular plants, 28 ferns and fern allies, 4 gymnosperms, and 459 angiosperms 244 dicots and 215 monocots. An additional 100 species are designated “watch” status (see “Special Plants - Watch List”) with the potential of becoming species of special concern and include 2 fern and fern allies, 54 dicots and 44 monocots. This list is designated for the primary purposes of : 1) in environmental assessments, “flagging” of sensitive species that may be negatively affected by proposed actions; 2) determination of protection priorities of natural areas that contain such species; and 3) determination of priorities of inventory and protection for these plants, including the proposed listing of species for federal protection. GLOBAL STATE FEDERAL SPECIES NAME COMMON NAME RANK RANK STATUS BRYOPSIDA Callicladium haldanianum Callicladium Moss G5 SNR Leptobryum pyriforme Leptobryum Moss G5 SNR Rhodobryum roseum Rose Moss G5 S1? Trachyxiphium heteroicum Trachyxiphium Moss G2? S1? EQUISETOPSIDA Equisetum arvense Field Horsetail G5 S1S2 FILICOPSIDA Adiantum capillus-veneris Southern Maidenhair-fern G5 S2 Asplenium -

ACANTHACEAE 爵床科 Jue Chuang Ke Hu Jiaqi (胡嘉琪 Hu Chia-Chi)1, Deng Yunfei (邓云飞)2; John R
ACANTHACEAE 爵床科 jue chuang ke Hu Jiaqi (胡嘉琪 Hu Chia-chi)1, Deng Yunfei (邓云飞)2; John R. I. Wood3, Thomas F. Daniel4 Prostrate, erect, or rarely climbing herbs (annual or perennial), subshrubs, shrubs, or rarely small trees, usually with cystoliths (except in following Chinese genera: Acanthus, Blepharis, Nelsonia, Ophiorrhiziphyllon, Staurogyne, and Thunbergia), isophyllous (leaf pairs of equal size at each node) or anisophyllous (leaf pairs of unequal size at each node). Branches decussate, terete to angular in cross-section, nodes often swollen, sometimes spinose with spines derived from reduced leaves, bracts, and/or bracteoles. Stipules absent. Leaves opposite [rarely alternate or whorled]; leaf blade margin entire, sinuate, crenate, dentate, or rarely pinnatifid. Inflo- rescences terminal or axillary spikes, racemes, panicles, or dense clusters, rarely of solitary flowers; bracts 1 per flower or dichasial cluster, large and brightly colored or minute and green, sometimes becoming spinose; bracteoles present or rarely absent, usually 2 per flower. Flowers sessile or pedicellate, bisexual, zygomorphic to subactinomorphic. Calyx synsepalous (at least basally), usually 4- or 5-lobed, rarely (Thunbergia) reduced to an entire cupular ring or 10–20-lobed. Corolla sympetalous, sometimes resupinate 180º by twisting of corolla tube; tube cylindric or funnelform; limb subactinomorphic (i.e., subequally 5-lobed) or zygomorphic (either 2- lipped with upper lip subentire to 2-lobed and lower lip 3-lobed, or rarely 1-lipped with 3 lobes); lobes ascending or descending cochlear, quincuncial, contorted, or open in bud. Stamens epipetalous, included in or exserted from corolla tube, 2 or 4 and didyna- mous; filaments distinct, connate in pairs, or monadelphous basally via a sheath (Strobilanthes); anthers with 1 or 2 thecae; thecae parallel to perpendicular, equally inserted to superposed, spherical to linear, base muticous or spurred, usually longitudinally dehis- cent; staminodes 0–3, consisting of minute projections or sterile filaments. -

BIOLOGICAL Evaluation Analysis Uint 23 9/13/2010
BIOLOGICAL Evaluation Analysis Uint 23 9/13/2010 BIOLOGICAL EVALUATION OF FOREST MANAGEMENT ACTIVITIES PROPOSED FOR ANALYSIS UNIT 23 (Compartments 253, 254, 255, 256, 257 and 259) Gloster and Crosby Quadrangles USDA Forest Service Southern Region (8) National Forests in Mississippi Homochitto National Forest Homochitto Ranger District Mississippi Prepared by: Kenneth L. Gordon _____________________________ Date____________________ Wildlife Biologist, USDA Forest Service Concurred by: KATHY W. LUNCEFORD _____________________ Date____________________ Environmental Coordinator for Mississippi, US Fish and Wildlife Service Homochitto Ranger District Page 1 BIOLOGICAL Evaluation Analysis Uint 23 9/13/2010 Introduction This Biological Evaluation (BE) documents the likely impacts on proposed, endangered, threatened, and sensitive (TES) species from forest management activities proposed for Analysis Unit 23. AU 23 is located in Amite and Wilkinson counties, Mississippi and is part of the Foster Creek subwatershed in the Homochitto River basin.. As a result of a recent court decision, Forest Plan Amendment # 16 and the Region 8 Supplement to FSM 2670 are no longer in effect. This BE follows the process used to decide when to inventory for TES species that is consistent with the requirement found in the Vegetation Management EIS for the Coastal Plain and Piedmont. This BE is in accordance with direction given in Forest Service Manual (FSM) 2672 to meet the 1989 Vegetation Management standard. As part of the NEPA decision making process, the BE provides a review of Forest Service (FS) activities in sufficient detail to determine how an action will affect any TES species. TES species, taken from both state and federal lists, are species whose viability is most likely to be put at risk from management actions. -

Genus Ruellia: Pharmacological and Phytochemical Importance in Ethnopharmacology
Acta Poloniae Pharmaceutica ñ Drug Research, Vol. 72 No. 5 pp. 821ñ827, 2015 ISSN 0001-6837 Polish Pharmaceutical Society REVIEW GENUS RUELLIA: PHARMACOLOGICAL AND PHYTOCHEMICAL IMPORTANCE IN ETHNOPHARMACOLOGY KHURRAM AFZAL1*, MUHAMMAD UZAIR1, BASHIR AHMAD CHAUDHARY1, ASHFAQ AHMAD2, SAMINA AFZAL1 and MALIK SAADULLAH1 1Faculty of Pharmacy, Bahauddin Zakariya University, Multan, Pakistan 2School of Pharmaceutical Sciences, Universiti Sains Malaysia, 11800, Pulau Penang, Malaysia Abstract: Ruellia is a genus of flowering plants commonly known as Ruellias or Wild Petunias which belongs to the family Acanthaceae. It contains about 250 genera and 2500 species. Most of these are shrubs, or twining vines; some are epiphytes. Only a few species are dis- tributed in temperate regions. They are distributed in Indonesia and Malaysia, Africa, Brazil, Central America and Pakistan. Some of these are used as medicinal plants. Many species of the genus has antinociceptive, antioxidant, analgesic, antispasmolytic, antiulcer, antidiabet- ic and anti-inflammatory properties. The phytochemicals constituents: glycosides, alkaloids, flavonoids and triterpenoids are present. The genus has been traditionally claimed to be used for the treatment of flu, asthma, fever, bronchitis, high blood pressure, eczema, and dia- betes. The objective of this review article is to summarize all the pharmacological and phytochemical evaluations or investigations to find area of gap and endorse this genus a step towards commercial drug. Hence, further work required is to isolate -

The Draft Genome of Ruellia Speciosa (Beautiful Wild Petunia: Acanthaceae) Yongbin Zhuang1,2 and Erin A
Dna Research, 2017, 24(2), 179–192 doi: 10.1093/dnares/dsw054 Advance Access Publication Date: 27 January 2017 Full Paper Full Paper The draft genome of Ruellia speciosa (Beautiful Wild Petunia: Acanthaceae) Yongbin Zhuang1,2 and Erin A. Tripp1,2,* 1Department of Ecology and Evolutionary Biology, University of Colorado, UCB 334, Boulder, CO 80309, USA and 2Museum of Natural History, University of Colorado, UCB 350, Boulder, CO 80309, USA *To whom correspondence should be addressed. Email: [email protected] Edited by Dr. Sachiko Isobe Received 18 July 2016; Editorial decision 16 November 2016; Accepted 17 November 2016 Abstract The genus Ruellia (Wild Petunias; Acanthaceae) is characterized by an enormous diversity of floral shapes and colours manifested among closely related species. Using Illumina platform, we reconstructed the draft genome of Ruellia speciosa, with a scaffold size of 1,021 Mb (or 1.02 Gb) and an N50 size of 17,908 bp, spanning 93% of the estimated genome (1.1 Gb). The draft assembly predicted 40,124 gene models and phylogenetic analyses of four key en- zymes involved in anthocyanin colour production [flavanone 3-hydroxylase (F3H), flavonoid 30- hydroxylase (F30H), flavonoid 30,50-hydroxylase (F3050H), and dihydroflavonol 4-reductase (DFR)] found that most angiosperms here sampled harboured at least one copy of F3H, F30H, and DFR. In contrast, fewer than one-half (but including R. speciosa) harboured a copy of F3050H, support- ing observations that blue flowers and/or fruits, which this enzyme is required for, are less com- mon among flowering plants. Ka/Ks analyses of duplicated copies of F30H and DFR in R.